J Vet Sci.
2017 Dec;18(4):507-514. 10.4142/jvs.2017.18.4.507.
Quantitative evaluation of renal parenchymal perfusion using contrast-enhanced ultrasonography in renal ischemia-reperfusion injury in dogs
- Affiliations
-
- 1College of Veterinary Medicine, Chonnam National University, Gwangju 61186, Korea. imsono@chonnam.ac.kr
- KMID: 2398510
- DOI: http://doi.org/10.4142/jvs.2017.18.4.507
Abstract
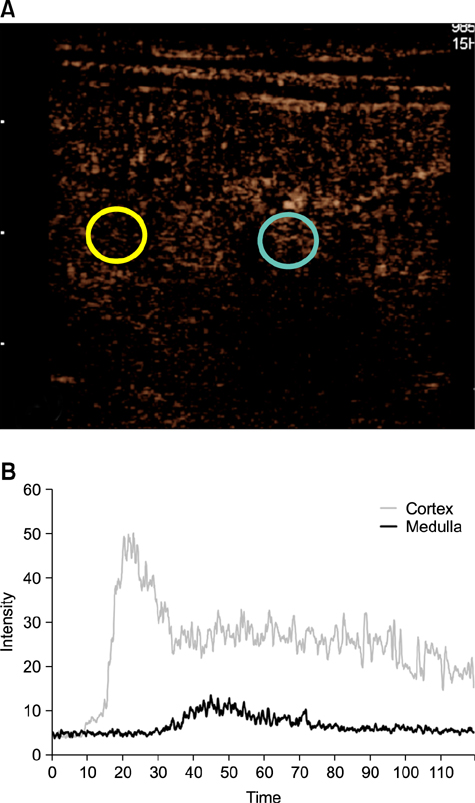
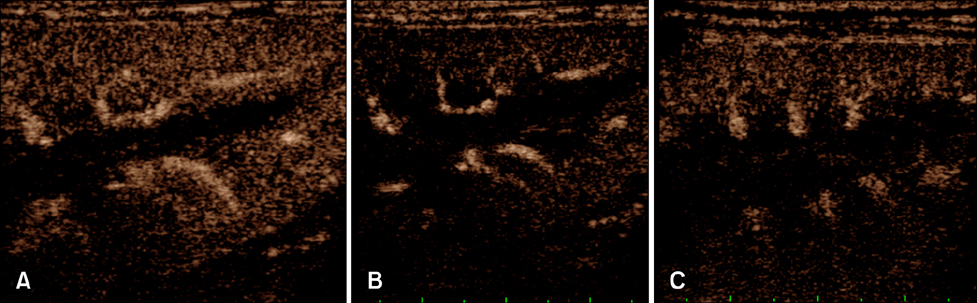
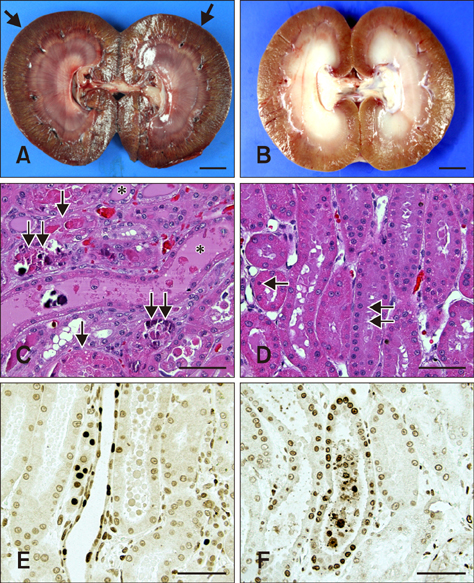
- This study evaluated whether renal perfusion changes can be noninvasively estimated by using contrast-enhanced ultrasonography (CEUS) in renal ischemia-reperfusion injury and investigated the correlation between renal perfusion measured by CEUS and necrosis and apoptosis of renal tubular epithelial cells. In six dogs with experimentally induced renal ischemia-reperfusion injury, changes in time to peak intensity, peak intensity, and area under the curve were measured on CEUS. Peak intensity and area under the curve of the renal cortex began to decrease on day 1 (about 20% lower than baseline) and reached the lowest levels (about 50% of baseline) on day 4. They then gradually increased until day 10, at which time peak intensity was about 87% and area under the curve was about 95% of baseline; neither fully recovered. Both parameters were strongly correlated with the necrosis scores on histopathologic examination on day 4 (r = −0.810 of peak intensity and r = −0.886 of area under the curve). CEUS allowed quantitative evaluation of perfusion changes in acute renal ischemia-reperfusion injury, and CEUS results were correlated with renal tubular damage on histopathologic examination. Thus, CEUS could be a noninvasive, quantitative diagnostic method for determining progress of renal ischemia-reperfusion injury.
Keyword
MeSH Terms
Figure
Reference
-
1. Adachi T, Sugiyama N, Yagita H, Yokoyama T. Renal atrophy after ischemia-reperfusion injury depends on massive tubular apoptosis induced by TNFα in the later phase. Med Mol Morphol. 2014; 47:213–223.
Article2. Aronson S, Thistlethwaite RJ, Walker R, Feinstein SB, Roizen MF. Safety and feasibility of renal blood flow determination during kidney transplant surgery with perfusion ultrasonography. Anesth Analg. 1995; 80:353–359.
Article3. Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002; 11:301–308.
Article4. Brown ED, Chen MYM, Wolfman NT, Ott DJ, Watson NE Jr. Complications of renal transplantation: evaluation with US and radionuclide imaging. Radiographics. 2000; 20:607–622.
Article5. Chow L, Sommer FG, Huang J, Li KC. Power Doppler imaging and resistance index measurement in the evaluation of acute renal transplant rejection. J Clin Ultrasound. 2001; 29:483–490.
Article6. Christensson A. Renovascular disease and renal insufficiency — diagnosis and treatment. Scand J Urol Nephrol. 1999; 33:400–405.
Article7. Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015; 34:3–18.
Article8. El Morsy EM, Ahmed MAE, Ahmed AAE. Attenuation of renal ischemia/reperfusion injury by açaí extract preconditioning in a rat model. Life Sci. 2015; 123:35–42.
Article9. Forsberg F, Basude R, Liu JB, Alessandro J, Shi WT, Rawool NM, Goldberg BB, Wheatley MA. Effect of filling gases on the backscatter from contrast microbubbles: theory and in vivo measurements. Ultrasound Med Biol. 1999; 25:1203–1211.
Article10. Heffron T, Gadowski G, Buckingham F, Salciunas P, Thistlethwaite JR Jr, Stuart FP. Laser Doppler blood flow measurement as a predictor of viability of renal allografts. Curr Surg. 1990; 47:431–432.11. Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 2005; 67:321–332.
Article12. Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983; 35:198–204.
Article13. Jimenez C, Lopez MO, Gonzalez E, Selgas R. Ultrasonography in kidney transplantation: values and new developments. Transplant Rev (Orlando). 2009; 23:209–213.
Article14. Kasap B, Soylu A, Türkmen M, Kavukcu S. Relationship of increased renal cortical echogenicity with clinical and laboratory findings in pediatric renal disease. J Clin Ultrasound. 2006; 34:339–342.
Article15. Kennedy SE, Erlich JH. Murine renal ischaemia-reperfusion injury. Nephrology (Carlton). 2008; 13:390–396.16. Kim JM, Lee JY, Jeong SM, Park CS, Kim MC. Attenuation of renal ischemia-reperfusion (I/R) injury by ascorbic acid in the canine nephrotomy. J Vet Clin. 2010; 27:553–558.17. Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, Racusen L, Rabb H. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010; 298:F1472–F1483.
Article18. Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005; 365:417–430.
Article19. Lechevallier E, Dussol B, Luccioni A, Thirion X, Vacher-Copomat H, Jaber K, Brunet P, Leonetti F, Lavelle O, Coulange C, Berland Y. Posttransplantation acute tubular necrosis: risk factors and implications for graft survival. Am J Kidney Dis. 1998; 32:984–991.
Article20. Lee JI, Son HY, Jeong SM, Kim MC. Amelioration effects of irrigation-aspiration on renal ischemia-reperfusion injury in canine model. J Vet Clin. 2008; 25:257–262.21. Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996; 271:477–488.
Article22. Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002; 15:396–403.
Article23. Liu AS, Xie JX. Functional evaluation of normothermic ischemia and reperfusion injury in dog kidney by combining MR diffusion-weighted imaging and Gd-DTPA enhanced first-pass perfusion. J Magn Reson Imaging. 2003; 17:683–693.
Article24. Nguan CYC, Guan Q, Gleave ME, Du C. Promotion of cell proliferation by clusterin in the renal tissue repair phase after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014; 306:F724–F733.
Article25. Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996; 24:25–29.
Article26. Park SB, Kim JK, Cho KS. Complications of renal transplantation: ultrasonographic evaluation. J Ultrasound Med. 2007; 26:615–633.27. Schwenger V, Korosoglou G, Hinkel UP, Morath C, Hansen A, Sommerer C, Dikow R, Hardt S, Schmidt J, Kücherer H, Katus HA, Zeier M. Real-time contrast-enhanced sonography of renal transplant recipients predicts chronic allograft nephropathy. Am J Transplant. 2006; 6:609–615.
Article28. Shuvy M, Nyska A, Beeri R, Abedat S, Gal-Moscovici A, Rajamannan NM, Lotan C. Histopathology and apoptosis in an animal model of reversible renal injury. Exp Toxicol Pathol. 2011; 63:303–306.
Article29. Szolar DH, Preidler K, Ebner F, Kammerhuber F, Horn S, Ratschek M, Ranner G, Petritsch P, Horina JH. Functional magnetic resonance imaging of human renal allografts during the post-transplant period: preliminary observations. Magn Reson Imaging. 1997; 15:727–735.
Article30. Trillaud H, Merville P, Tran Le Linh P, Palussière J, Potaux L, Grenier NP. Color Doppler sonography in early renal transplantation follow-up: resistive index measurements versus power Doppler sonography. AJR Am J Roentgenol. 1998; 171:1611–1615.
Article31. Zeisbrich M, Kihm LP, Drüschler F, Zeier M, Schwenger V. When is contrast-enhanced sonography preferable over conventional ultrasound combined with Doppler imaging in renal transplantation. Clin Kidney J. 2015; 8:606–614.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imaging Features of Gray-Scale and Contrast-Enhanced Color Doppler US for the Differentiation of Transient Renal Arterial Ischemia and Arterial Infarction
- US Features of Experimentally-induced Transient Ischemia and Infarct of Renal Segmental Artery of Rabbits
- Influence of ascorbic acid on BUN, creatinine, resistive index in canine renal ischemia-reperfusion injury
- Dynamic Contrast-Enhanced MR Imaging of Renal Ischemia-Reperfusion Injury
- Renal ischemia-reperfusion injury does not induce pulmonary dysfunction in sheep