Two-Track Medical Treatment Strategy According to the Clinical Scoring System for Chronic Rhinosinusitis
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. kicubi@daum.net
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 4Division of Allergy-Immunology, Department of Internal Medicine, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
- KMID: 2418047
- DOI: http://doi.org/10.4168/aair.2018.10.5.490
Abstract
- PURPOSE
The previously reported Japanese clinical scoring study (JESREC) suggests that chronic rhinosinusitis (CRS) can be divided into 4 subtypes according to the degree of eosinophilic CRS (ECRS) and offers the information regarding the prognosis of CRS to clinicians. However, this scoring system has not yet been validated by an immunological study and needs to provide treatment guidelines based on underlying immunologic profiles. We investigated the immunologic profile of each CRS subgroup according to the JESREC classification and suggest its clinical application.
METHODS
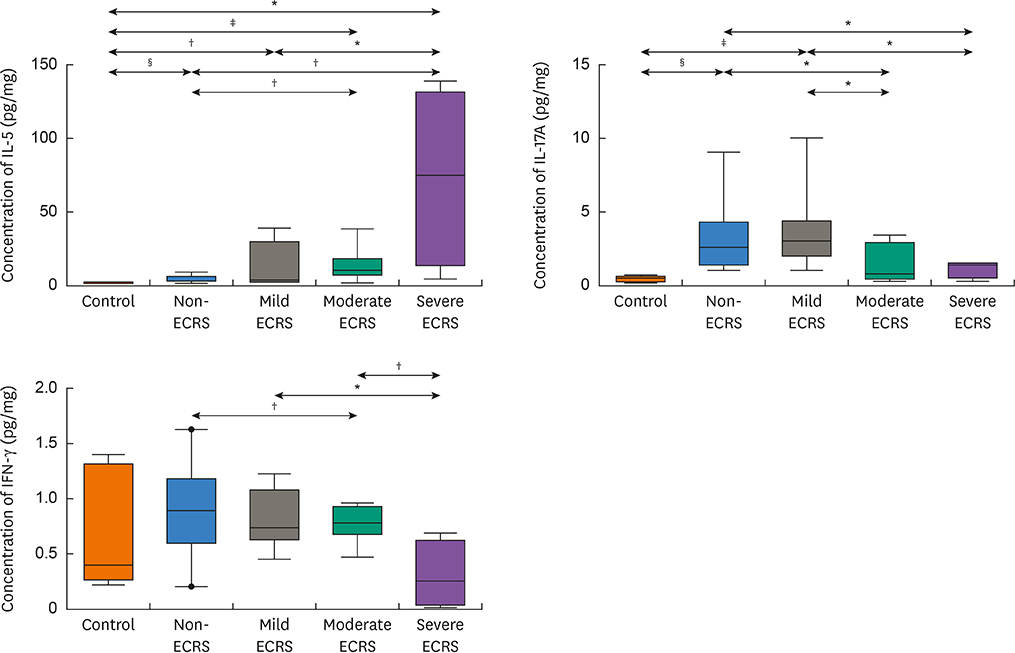
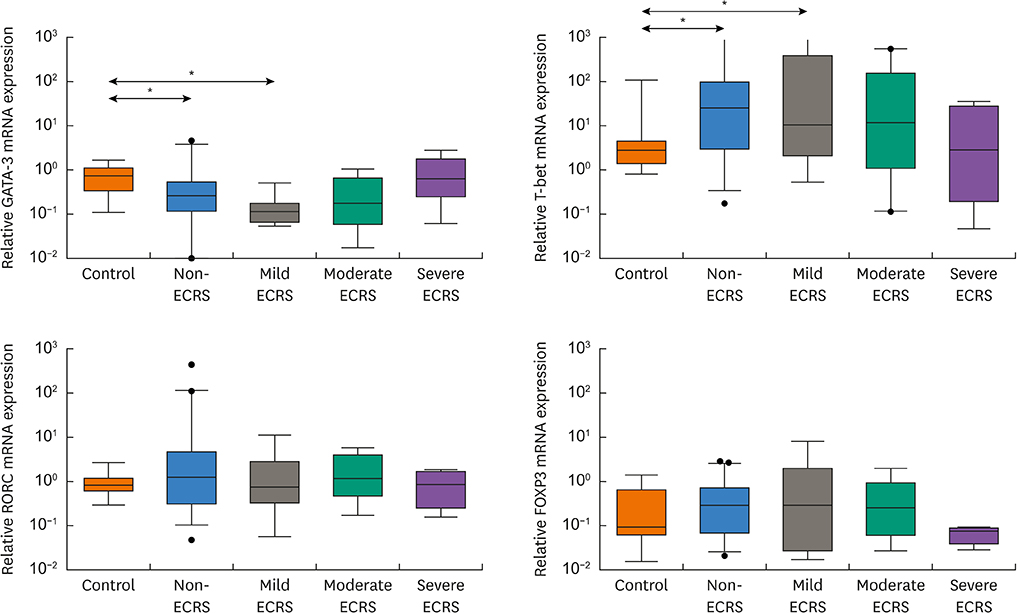
A total of 140 CRS patients and 20 control subjects were enrolled. All patients were classified into 4 groups according to the JESREC (non-, mild, moderate and severe ECRS). Nasal tissues were analyzed for mRNA expression of major cytokines (IL-5, IL-10, IL-13, IL-17A, IL-22, IL-23p19, IFN-γ, periostin, thymic stromal lymphopoietin [TSLP] and ST2), major chemokines (CCL11, CCL24, CXCL1 and CXCL2), transcription factors (T-bet, GATA3, RORC and FOXP3) and COL1A1 for type I collagen. Protein levels of 3 major cytokines (IL-5, IL-17A and IFN-γ) were also measured by multiplex immunoassay. Principal component analysis (PCA) was conducted to investigate the overall profile of multiple mediators.
RESULTS
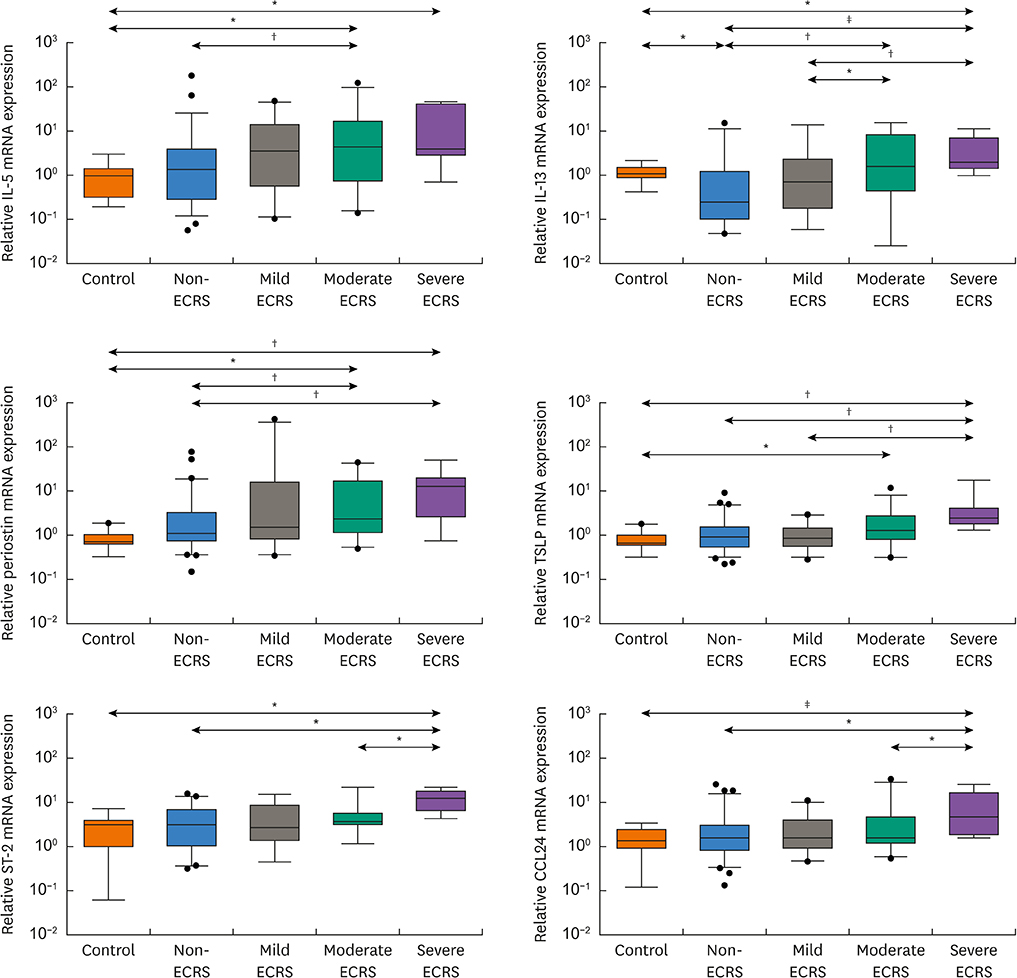
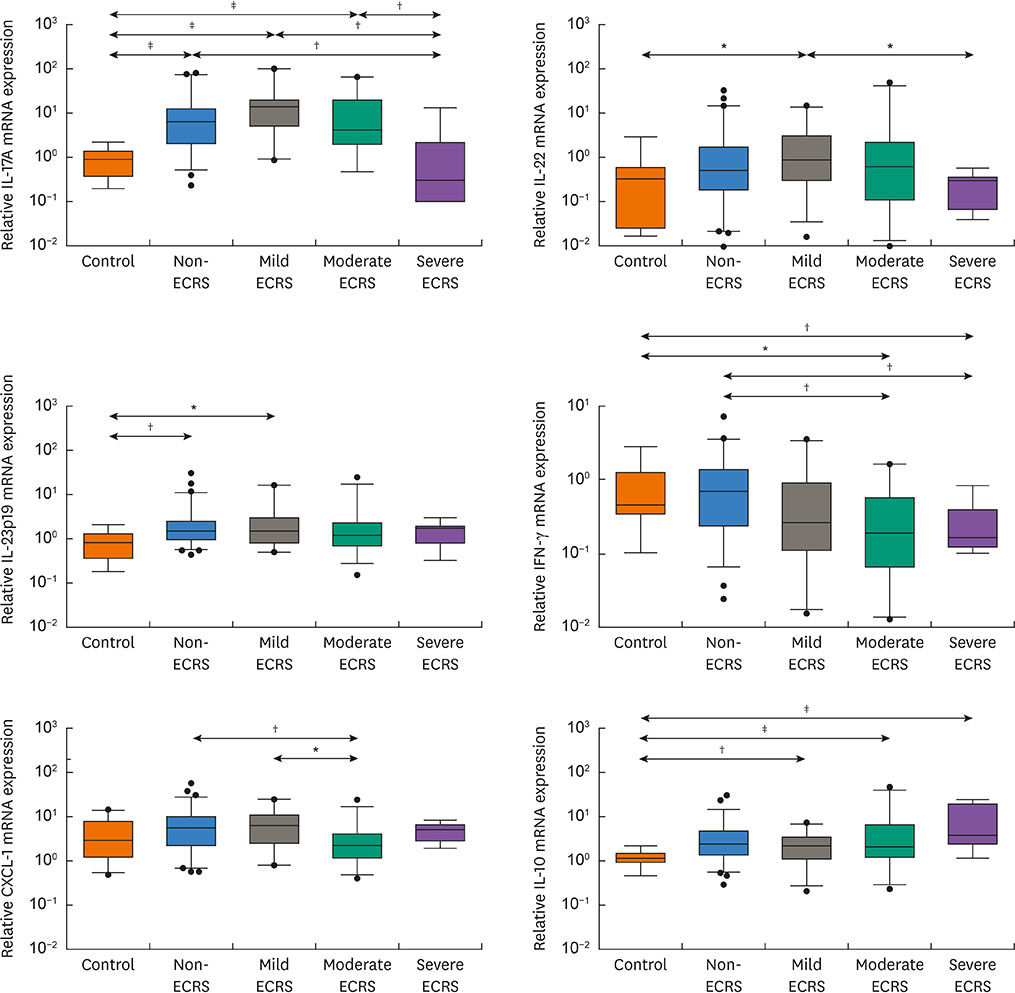
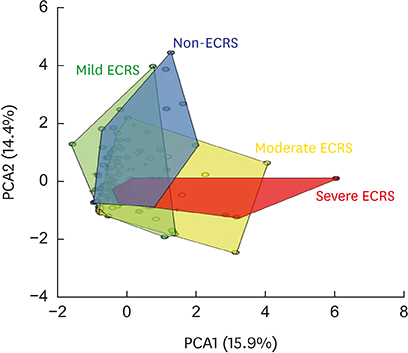
The moderate/severe ECRS showed up-regulation of type 2-related mediators (IL-5, IL-13, periostin, TSLP and ST-2), whereas INF-γ (type 1 cytokine) and CXCL1 (neutrophil chemokine) expressions were increased in non-/mild ECRS compared with moderate/severe ECRS. The JESREC classification reflected an immunological endotype. In PCA data, PCA1 indicates a relative type 2 profile, whereas PCA2 represents a type 1/type 17-related profile. In this analysis, mild ECRS was indistinguishable from non-ECRS, whereas moderate to severe ECRS showed a distinct distribution compared with non-ECRS. The JESREC classification could be divided into 2 categories, non-/mild vs. moderate/severe ECRS based on underlying immunological analyses.
CONCLUSIONS
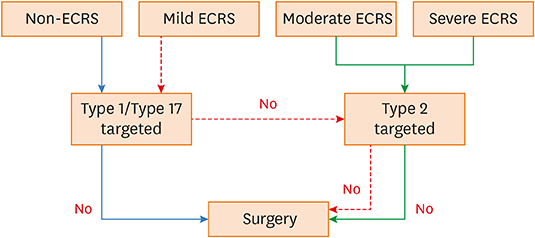
The CRS clinical scoring system from the JESREC study reflects an inflammatory endotype. However, the immunologic profile of mild ECRS was similar to that of non-ECRS. Therefore, we propose type 2-targeted medical treatment for moderate to severe ECRS and type 1/type 17-targeted for non-ECRS and mild ECRS as the first treatment option.
Keyword
MeSH Terms
-
Asian Continental Ancestry Group
Chemokines
Classification
Collagen Type I
Cytokines
Eosinophils
Humans
Immunoassay
Interleukin-10
Interleukin-13
Interleukin-17
Interleukin-23 Subunit p19
Nasal Polyps
Passive Cutaneous Anaphylaxis
Principal Component Analysis
Prognosis
Rhinitis
RNA, Messenger
Sinusitis
Transcription Factors
Up-Regulation
Chemokines
Collagen Type I
Cytokines
Interleukin-10
Interleukin-13
Interleukin-17
Interleukin-23 Subunit p19
RNA, Messenger
Transcription Factors
Figure
Cited by 6 articles
-
Comparison Between Signature Cytokines of Nasal Tissues in Subtypes of Chronic Rhinosinusitis
Dong-Kyu Kim, Kyoung Mi Eun, Min-Kyung Kim, Deuktae Cho, Sun A Han, Sang-Yoon Han, Yuju Seo, Dong-Han Lee, Seong Ho Cho, Dae Woo Kim
Allergy Asthma Immunol Res. 2019;11(2):201-211. doi: 10.4168/aair.2019.11.2.201.Chinese Society of Allergy and Chinese Society of Otorhinolaryngology-Head and Neck Surgery Guideline for Chronic Rhinosinusitis
Zheng Liu, Jianjun Chen, Lei Cheng, Huabin Li, Shixi Liu, Hongfei Lou, Jianbo Shi, Ying Sun, Dehui Wang, Chengshuo Wang, Xiangdong Wang, Yongxiang Wei, Weiping Wen, Pingchang Yang, Qintai Yang, Gehua Zhang, Yuan Zhang, Changqing Zhao, Dongdong Zhu, Li Zhu, Fenghong Chen, Yi Dong, Qingling Fu, Jingyun Li, Yanqing Li, Chengyao Liu, Feng Liu, Meiping Lu, Yifan Meng, Jichao Sha, Wenyu She, Lili Shi, Kuiji Wang, Jinmei Xue, Luoying Yang, Min Yin, Lichuan Zhang, Ming Zheng, Bing Zhou, Luo Zhang
Allergy Asthma Immunol Res. 2020;12(2):176-237. doi: 10.4168/aair.2020.12.2.176.Elastase-Positive Neutrophils Are Associated With Refractoriness of Chronic Rhinosinusitis With Nasal Polyps in an Asian Population
Dong-Kyu Kim, Jin Youp Kim, Young Eun Han, Joon Kon Kim, Hee-Suk Lim, Kyoung Mi Eun, Seung Koo Yang, Dae Woo Kim
Allergy Asthma Immunol Res. 2020;12(1):42-55. doi: 10.4168/aair.2020.12.1.42.Biomarkers in Chronic Rhinosinusitis with Nasal Polyp: Personalized Medicine Based on Endotype
Yoonjae Song, Sung-Woo Cho
Korean J Otorhinolaryngol-Head Neck Surg. 2019;62(8):427-434. doi: 10.3342/kjorl-hns.2019.00332.Clinical Characteristics of Chronic Rhinosinusitis With Nasal Polyp According to Histopathological Endotypes and Staining Method for Neutrophilic Polyp Classification and Its Clinical Implication
Hyoyeon Kim, Shin Hyuk Yoo, Kwang Hyun Byun, Ji-Hun Mo
Korean J Otorhinolaryngol-Head Neck Surg. 2024;67(2):79-86. doi: 10.3342/kjorl-hns.2023.00332.Analysis of Clinical Characteristics and Post-Treatment Prognosis of Eosinophilic and Neutrophilic Chronic Rhinosinusitis With Nasal Polyps in Korean Patients
Chae-Young Kim, Kwang-Hyun Byun, Jun-Sang Bae, Eun-Hee Kim, Shin-Hyuk Yoo, Ji-Hun Mo
Korean J Otorhinolaryngol-Head Neck Surg. 2024;67(6):328-335. doi: 10.3342/kjorl-hns.2023.00857.
Reference
-
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.
Article2. Brandsted R, Sindwani R. Impact of depression on disease-specific symptoms and quality of life in patients with chronic rhinosinusitis. Am J Rhinol. 2007; 21:50–54.
Article3. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017; 127:550–555.
Article4. Stein NR, Jafari A, DeConde AS. Revision rates and time to revision following endoscopic sinus surgery: a large database analysis. Laryngoscope. 2018; 128:31–36.
Article5. Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015; 70:995–1003.6. Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006; 61:1275–1279.
Article7. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289.
Article8. Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008; 121:1435–1441. 1441.e1–1441.e3.
Article9. Kim DK, Park MH, Chang DY, Eun KM, Shin HW, Mo JH, et al. MBP-positive and CD11c-positive cells are associated with different phenotypes of Korean patients with non-asthmatic chronic rhinosinusitis. PLoS One. 2014; 9:e111352.
Article10. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968.
Article11. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; 124:478–484. 484.e1–484.e2.
Article12. Derycke L, Eyerich S, Van Crombruggen K, Pérez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014; 9:e97581.
Article13. Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010; 142:64–71.
Article14. Prokopakis E, Vlastos I, Pant H, Ferguson BJ. Chronic rhinosinusitis: observation or treatment. Curr Opin Allergy Clin Immunol. 2013; 13:31–36.15. Klimek L, Dollner R, Pfaar O, Mullol J. Aspirin desensitization: useful treatment for chronic rhinosinusitis with nasal polyps (CRSwNP) in aspirin-exacerbated respiratory disease (AERD)? Curr Allergy Asthma Rep. 2014; 14:441.
Article16. Lee JY, Simon RA, Stevenson DD. Selection of aspirin dosages for aspirin desensitization treatment in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2007; 119:157–164.
Article17. Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003; 111:180–186.
Article18. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137:1449–1456.e4.
Article19. Liao B, Liu JX, Li ZY, Zhen Z, Cao PP, Yao Y, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. Forthcoming. 2018.
Article20. Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010; 59:239–245.
Article21. Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013; 123:E1–E9.
Article22. Nakayama T, Yoshikawa M, Asaka D, Okushi T, Matsuwaki Y, Otori N, et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology. 2011; 49:392–396.
Article23. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138:1344–1353.24. Shi LL, Song J, Xiong P, Cao PP, Liao B, Ma J, et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2014; 190:628–638.
Article25. Wang X, Li ZY, Jiang WX, Liao B, Zhai GT, Wang N, et al. The activation and function of IL-36 γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2018; 141:1646–1658.26. Seshadri S, Rosati M, Lin DC, Carter RG, Norton JE, Choi AW, et al. Regional differences in the expression of innate host defense molecules in sinonasal mucosa. J Allergy Clin Immunol. 2013; 132:1227–1230.e5.
Article27. Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015; 192:682–694.
Article28. Kim DW, Cho SH. Emerging endotypes of chronic rhinosinusitis and its application to precision medicine. Allergy Asthma Immunol Res. 2017; 9:299–306.
Article29. Aukema AA, Mulder PG, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. J Allergy Clin Immunol. 2005; 115:1017–1023.
Article30. Martinez-Devesa P, Patiar S. Oral steroids for nasal polyps. Cochrane Database Syst Rev. 2011; (7):CD005232.
Article31. Kowalski ML. Oral and nasal steroids for nasal polyps. Curr Allergy Asthma Rep. 2011; 11:187–188.
Article32. Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010; 65:384–390.
Article33. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007; 104:15858–15863.
Article34. Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013; 131:1479–1490.35. Gurrola J 2nd, Borish L. Chronic rhinosinusitis: endotypes, biomarkers, and treatment response. J Allergy Clin Immunol. 2017; 140:1499–1508.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical treatment according to phenotypes of chronic rhinosinusitis
- The Clinical Significance of Lund-Mackay CT Staging System in Assessing the Severity of Chronic Rhinosinusitis
- Clinical Characteristics and Treatment of Fungal Rhinosinusitis
- Characteristics of Computed Tomography in Chronic Rhinosinusitis with Asthma
- Tissue Remodeling in Rhinosinusitis