Korean J Pain.
2010 Mar;23(1):1-10.
Superoxide and Nitric Oxide Involvement in Enhancing of N-methyl-D-aspartate Receptor-Mediated Central Sensitization in the Chronic Post-ischemia Pain Model
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Kyungpook National University School of Medicine, Daegu, Korea. kwakkh@knu.ac.kr
Abstract
- BACKGROUND
Recent studies indicate that reactive oxygen species (ROS) are involved in persistent pain, including neuropathic and inflammatory pain. Since the data suggest that ROS are involved in central sensitization, the present study examines the levels of activated N-methyl-D-aspartate (NMDA) receptors in the dorsal horn after an exogenous supply of three antioxidants in rats with chronic post-ischemia pain (CPIP). This serves as an animal model of complex regional pain syndrome type-I induced by hindpaw ischemia/reperfusion injury.
METHODS
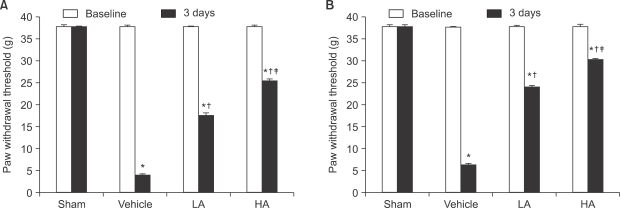
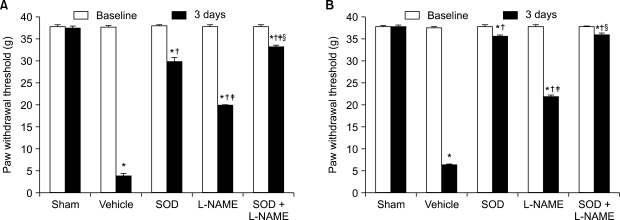
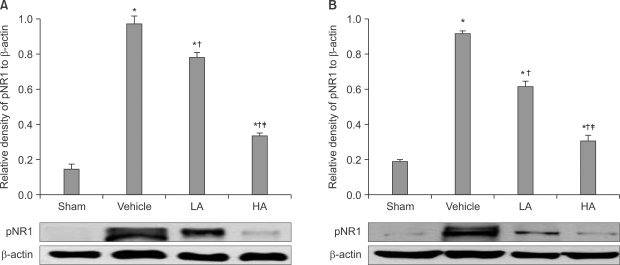
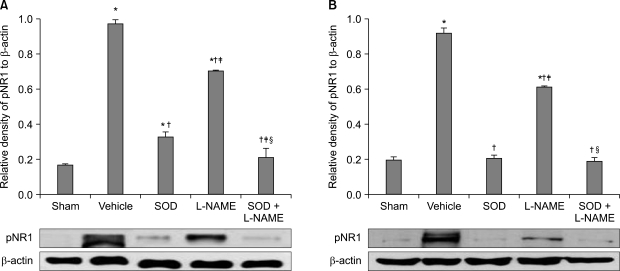
The application of tight-fitting O-rings for a period of three hours produced CPIP in male Sprague-Dawley rats. Allopurinol 4 mg/kg, allopurinol 40 mg/kg, superoxide dismutase (SOD) 4,000 U/kg, N-nitro-L-arginine methyl ester (L-NAME) 10 mg/kg and SOD 4,000 U/kg plus L-NAME 10 mg/kg were administered intraperitoneally just after O-ring application and on the first and second days after reperfusion. Mechanical allodynia was measured, and activation of the NMDA receptor subunit 1 (pNR1) of the lumbar spinal cord (L4-L6) was analyzed by the Western blot three days after reperfusion.
RESULTS
Allopurinol reduced mechanical allodynia and attenuated the enhancement of spinal pNR1 expression in CPIP rats. SOD and L-NAME also blocked spinal pNR1 in accordance with the reduced mechanical allodynia in rats with CPIP.
CONCLUSION
The present data suggest the contribution of superoxide, produced via xanthine oxidase, and the participation of superoxide and nitric oxide as a precursor of peroxynitrite in NMDA mediated central sensitization. Finally, the findings support a therapeutic potential for the manipulation of superoxide and nitric oxide in ischemia/reperfusion related pain conditions.
Keyword
MeSH Terms
-
Allopurinol
Animals
Antioxidants
Blotting, Western
Central Nervous System Sensitization
Horns
Humans
Hyperalgesia
Inositol Phosphates
Male
Models, Animal
N-Methylaspartate
NG-Nitroarginine Methyl Ester
Nitric Oxide
Peroxynitrous Acid
Prostaglandins E
Rats
Rats, Sprague-Dawley
Reactive Oxygen Species
Reperfusion
Reperfusion Injury
Spinal Cord
Superoxide Dismutase
Superoxides
Xanthine Oxidase
Allopurinol
Antioxidants
Inositol Phosphates
N-Methylaspartate
NG-Nitroarginine Methyl Ester
Nitric Oxide
Peroxynitrous Acid
Prostaglandins E
Reactive Oxygen Species
Superoxide Dismutase
Superoxides
Xanthine Oxidase
Figure
Reference
-
1. Jänig W, Baron R. Complex regional pain syndrome is a disease of the central nervous system. Clin Auton Res. 2002; 12:150–164. PMID: 12269546.
Article2. Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004; 112:94–105. PMID: 15494189.
Article3. Kwak KH, Han CG, Lee SH, Jeon Y, Park SS, Kim SO, et al. Reactive oxygen species in rats with chronic postischemia pain. Acta Anaesthesiol Scand. 2009; 53:648–656. PMID: 19419360.
Article4. Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004; 111:116–124. PMID: 15327815.
Article5. Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL Jr. Antioxidants attenuate multiple phases of formalin induced nociceptive response in mice. Behav Brain Res. 2006; 173:211–216. PMID: 16919817.
Article6. Guedes RP, Bosco LD, Teixeira CM, Araújo AS, Llesuy S, Belló-Klein A, et al. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006; 31:603–609. PMID: 16770731.
Article7. Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006; 391:108–111. PMID: 16183198.
Article8. Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007; 131:262–271. PMID: 17317010.
Article9. Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000; 20:6989–6997. PMID: 10995844.
Article10. Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992; 12:3665–3670. PMID: 1326610.
Article11. Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005; 116:62–72. PMID: 15936881.
Article12. Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007; 18:570–580. PMID: 17202421.
Article13. Radovic M, Miloradovic Z, Popovic T, Mihailovic-Stanojevic N, Jovovic D, Tomovic M, et al. Allopurinol and enalapril failed to conserve urinary NOx and sodium in ischemic acute renal failure in spontaneously hypertensive rats. Am J Nephrol. 2006; 26:388–399. PMID: 16900002.
Article14. Lee WY, Lee SM. Synergistic protective effect of ischemic preconditioning and allopurinol on ischemia/reperfusion injury in rat liver. Biochem Biophys Res Commun. 2006; 349:1087–1093. PMID: 16959212.
Article15. Nemeth N, Lesznyak T, Szokoly M, Furka I, Miko I. Allopurinol prevents erythrocyte deformability impairing but not the hematological alterations after limb ischemia reperfusion in rats. J Invest Surg. 2006; 19:47–56. PMID: 16546929.
Article16. Albuquerque RG, Sanson AJ, Malangoni MA. Allopurinol protects enterocytes from hypoxia-induced apoptosis in vivo. J Trauma. 2002; 53:415–420. PMID: 12352473.
Article17. Levy D, Zochodne DW. Local nitric oxide synthase activity in a model of neuropathic pain. Eur J Neurosci. 1998; 10:1846–1855. PMID: 9751155.
Article18. Caban A, Oczkowicz G, Abdel-Samad O, Cierpka L. Influence of ischemic preconditioning and nitric oxide on microcirculation and the degree of rat liver injury in the model of ischemia and reperfusion. Transplant Proc. 2006; 38:196–198. PMID: 16504701.
Article19. Halliwell B, Gutteridge JC. Free radicals in biology and medicine. 2007. 4th ed. New York: Oxford University Press;p. 22.20. Salvemini D, Riley DP, Lennon PJ, Wang ZQ, Currie MG, Macarthur H, et al. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br J Pharmacol. 1999; 127:685–692. PMID: 10401559.
Article21. Xia ZF, Hollyoak M, Barrow RE, He F, Muller MJ, Herndon DN. Superoxide dismutase and leupeptin prevent delayed reperfusion injury in the rat small intestine during burn shock. J Burn Care Rehabil. 1995; 16:111–117. PMID: 7775503.
Article22. Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002; 282:C227–C241. PMID: 11788333.
Article23. Kim KW, Ha MJ, Jung KY, Kwak KH, Park SS, Lim DG. Reactive oxygen species and N-methyl-D-aspartate receptor-mediated central sensitization in hindlimb ischemia/reperfusion injury-induced neuropathic pain rats. Korean J Anesthesiol. 2009; 56:186–194.
Article24. Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001; 31:430–439. PMID: 11498276.
Article25. Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004; 309:869–878. PMID: 14988418.
Article26. Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, et al. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009; 29:159–168. PMID: 19129394.
Article27. Xu L, Mabuchi T, Katano T, Matsumura S, Okuda-Ashitaka E, Sakimura K, et al. Nitric oxide (NO) serves as a retrograde messenger to activate neuronal NO synthase in the spinal cord via NMDA receptors. Nitric Oxide. 2007; 17:18–24. PMID: 17548218.
Article28. Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, et al. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004; 111:96–103. PMID: 15327813.
Article29. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983; 306:686–688. PMID: 6656869.
Article30. Masu M, Nakajima Y, Moriyoshi K, Ishii T, Akazawa C, Nakanashi S. Molecular characterization of NMDA and metabotropic glutamate receptors. Ann N Y Acad Sci. 1993; 707:153–164. PMID: 9137550.
Article31. Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995; 34:1219–1237. PMID: 8570021.
Article32. Guix FX, Uribesalgo I, Coma M, Muñoz FJ. Physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005; 76:126–152. PMID: 16115721.33. Zhang XC, Zhang YQ, Zhao ZQ. Different roles of two nitric oxide activated pathways in spinal long-term potentiation of C-fiber-evoked field potentials. Neuropharmacology. 2006; 50:748–754. PMID: 16427664.
Article34. Duarte ID, dos Santos IR, Lorenzetti BB, Ferreira SH. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide cGMP pathway. Eur J Pharmacol. 1992; 217:225–227. PMID: 1330593.
Article35. Ferreira SH, Lorenzetti BB, Faccioli LH. Blockade of hyperalgesia and neurogenic oedema by topical application of nitroglycerin. Eur J Pharmacol. 1992; 217:207–209. PMID: 1425939.
Article36. Bulutcu F, Dogrul A, Güc MO. The involvement of nitric oxide in the analgesic effects of ketamine. Life Sci. 2002; 71:841–853. PMID: 12074943.
Article37. Granados-Soto V, Rufino MO, Gomes Lopes LD, Ferreira SH. Evidence for the involvement of the nitric oxide-cGMP pathway in the antinociception of morphine in the formalin test. Eur J Pharmacol. 1997; 340:177–180. PMID: 9537812.
Article38. Mixcoatl-Zecuatl T, Flores-Murrieta FJ, Granados-Soto V. The nitric oxide-cyclic GMP-protein kinase G-K+ channel pathway participates in the antiallodynic effect of spinal gabapentin. Eur J Pharmacol. 2006; 531:87–95. PMID: 16438951.
Article39. Ortiz MI, Castro-Olguin J, Peña-Samaniego N, Castañeda-Hernández G. Probable activation of the opioid receptor-nitric oxide-cyclic GMP-K+ channels pathway by codeine. Pharmacol Biochem Behav. 2005; 82:695–703. PMID: 16386786.
Article40. Ortiz MI, Granados-Soto V, Castañeda-Hernández G. The NO-cGMP-K+ channel pathway participates in the antinociceptive effect of diclofenac, but not of indomethacin. Pharmacol Biochem Behav. 2003; 76:187–195. PMID: 13679232.
Article41. de Moura RS, Rios AA, Santos EJ, Nascimento AB, de Castro Resende A, Neto ML, et al. Role of the NO-cGMP pathway in the systemic antinociceptive effect of clonidine in rats and mice. Pharmacol Biochem Behav. 2004; 78:247–253. PMID: 15219764.
Article42. Xanthos DN, Bennett GJ, Coderre TJ. Norepinephrine-induced nociception and vasoconstrictor hypersensitivity in rats with chronic post-ischemia pain. Pain. 2008; 137:640–651. PMID: 18079061.
Article43. Kim YC. Complex regional pain syndrome. Korean J Pain. 2004; 17(Suppl):104–108.
Article44. Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003; 23:1026–1040. PMID: 12574433.
Article45. Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, et al. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain. 2004; 110:299–309. PMID: 15275780.
Article46. Gordh T, Sharma HS. Chronic spinal nerve ligation induces microvascular permeability disturbances, astrocytic reaction, and structural changes in the rat spinal cord. Acta Neurochir Suppl. 2006; 96:335–340. PMID: 16671481.
Article47. Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979; 186:117–131. PMID: 447880.
Article48. Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999; 22:122–127. PMID: 10199637.
Article49. Donaldson LF. Unilateral arthritis: contralateral effects. Trends Neurosci. 1999; 22:495–496. PMID: 10530993.
Article50. Kwak KH, Jung KY, Choi JY, Ryu T, Yeo JS, Park SS, et al. Contralateral allodynia and central change in the chronic post-ischemic pain model rats. Korean J Anesthesiol. 2009; 56:419–424.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of superoxide on the development and maintenance of mechanical allodynia in a rat model of chronic post-ischemia pain
- Reactive oxygen species and N-methyl-D-aspartate receptor-mediated central sensitization in hindlimb ischemia/reperfusion injury-induced neuropathic pain rats
- Expression of nitric oxide synthase isoforms and N-methyl-D-aspartate receptor subunits according to transforming growth factor-beta1 administration after hypoxic-ischemic brain injury in neonatal rats
- A Case of Mixed Intravenous Infusion of Ketamine and Lidocaine in the Treatment of Post-herpetic Neuralgia
- Pulsed Radiofrequency Stimulation for Radicular Pain