Korean J Physiol Pharmacol.
2017 Sep;21(5):519-529. 10.4196/kjpp.2017.21.5.519.
Sodium butyrate has context-dependent actions on dipeptidyl peptidase-4 and other metabolic parameters
- Affiliations
-
- 1College of Pharmacy, Wonkwang University, Iksan 54538, Korea.
- 2College of Pharmacy, Chosun University, Gwangju 61452, Korea.
- 3Hanbang Body Fluid Research Center, Wonkwang University, Iksan 54538, Korea.
- 4College of Oriental Medicine and Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan 54538, Korea.
- 5Lee Gil Ya Cancer and Diabetes Institute, College of Medicine, Gachon University, Incheon 21999, Korea.
- 6Department of physiology, College of Medicine, Gachon University, Incheon 21999, Korea.
- 7Department of Internal Medicine, Gachon University Gil Medical Center, Incheon 21565, Korea. drhormone@naver.com
- 8Department of Internal Medicine, Gachon University College of Medicine, Incheon 21999, Korea.
- KMID: 2388697
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.519
Abstract
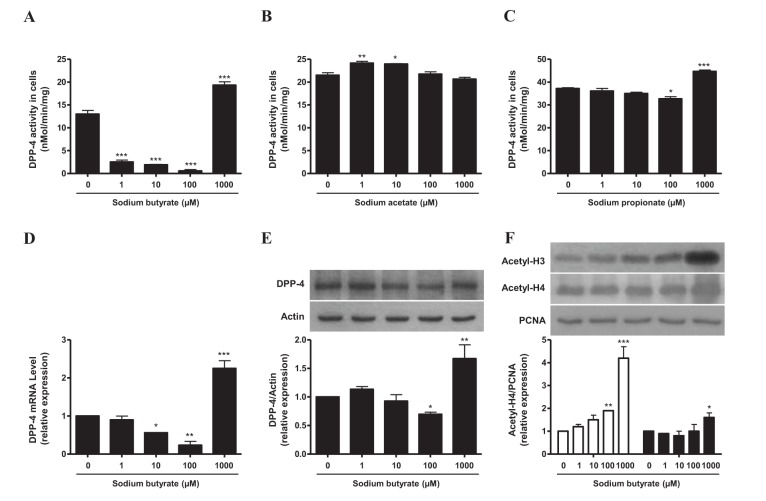
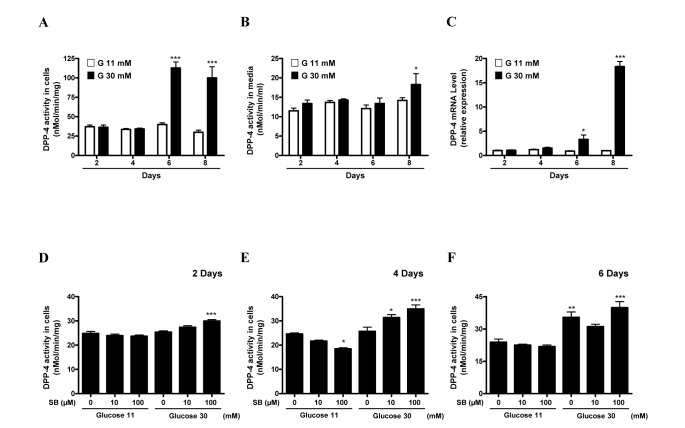
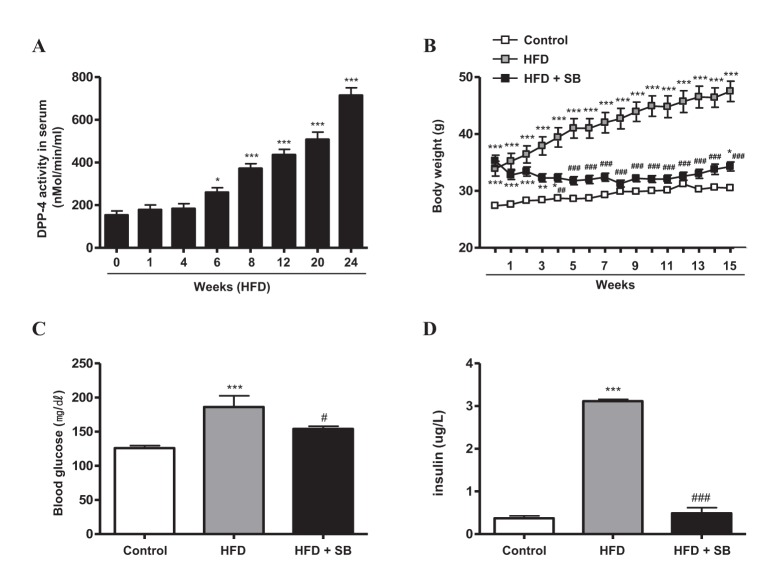
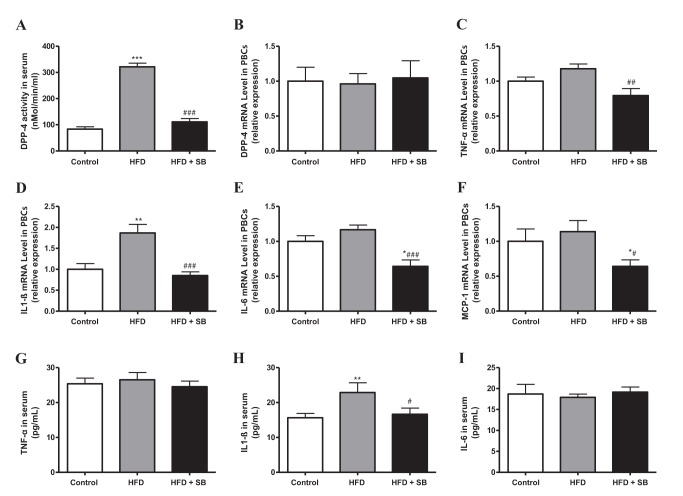
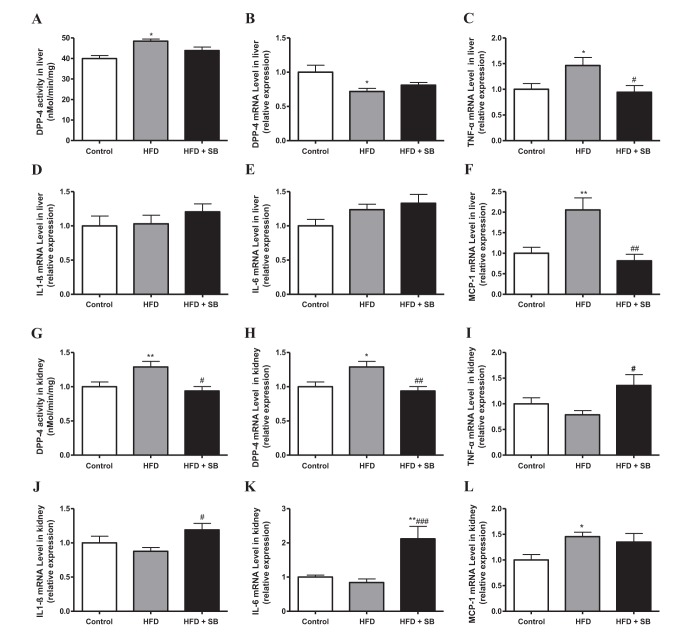
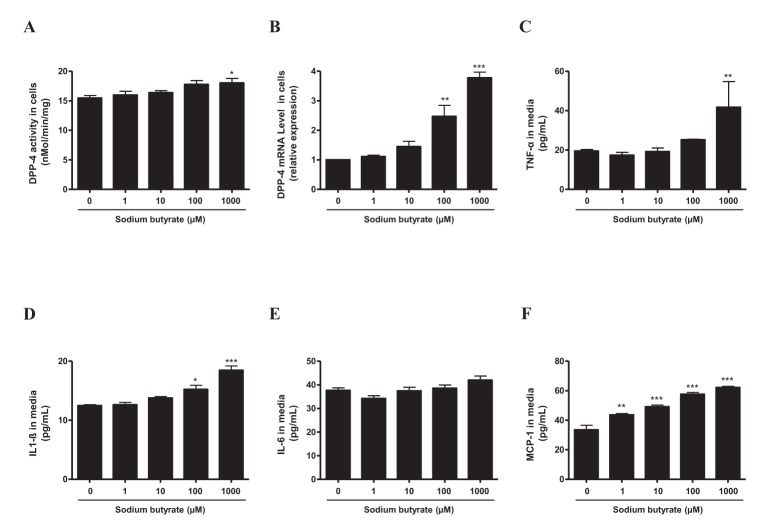
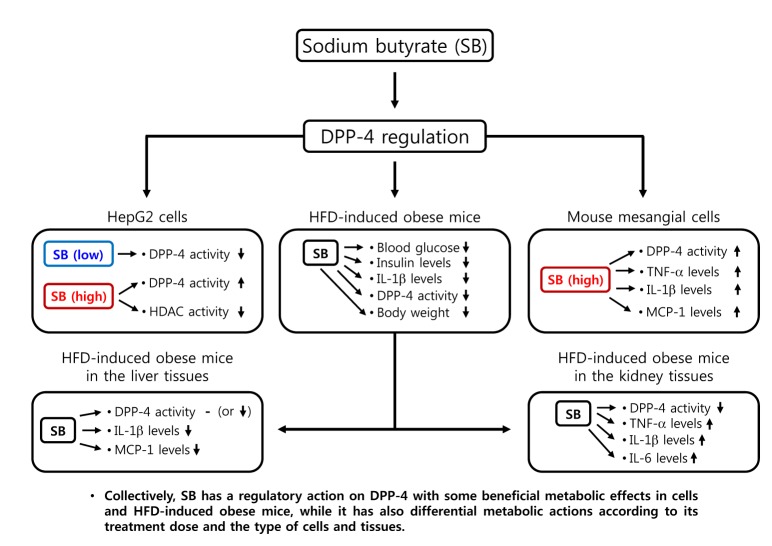
- Sodium butyrate (SB) has various metabolic actions. However, its effect on dipeptidyl peptidase 4 (DPP-4) needs to be studied further. We aimed to evaluate the metabolic actions of SB, considering its physiologically relevant concentration. We evaluated the effect of SB on regulation of DPP-4 and its other metabolic actions, both in vitro (HepG2 cells and mouse mesangial cells) and in vivo (high fat diet [HFD]-induced obese mice). Ten-week HFD-induced obese C57BL/6J mice were subjected to SB treatment by adding SB to HFD which was maintained for an additional 16 weeks. In HepG2 cells, SB suppressed DPP-4 activity and expression at sub-molar concentrations, whereas it increased DPP-4 activity at a concentration of 1,000 µM. In HFD-induced obese mice, SB decreased blood glucose, serum levels of insulin and IL-1β, and DPP-4 activity, and suppressed the increase in body weight. On the contrary, various tissues including liver, kidney, and peripheral blood cells showed variable responses of DPP-4 to SB. Especially in the kidney, although DPP-4 activity was decreased by SB in HFD-induced obese mice, it caused an increase in mRNA expression of TNF-α, IL-6, and IL-1β. The pro-inflammatory actions of SB in the kidney of HFD-induced obese mice were recapitulated by cultured mesangial cell experiments, in which SB stimulated the secretion of several cytokines from cells. Our results showed that SB has differential actions according to its treatment dose and the type of cells and tissues. Thus, further studies are required to evaluate its therapeutic relevance in metabolic diseases including diabetes and obesity.
MeSH Terms
-
Animals
Blood Cells
Blood Glucose
Body Weight
Butyric Acid*
Cytokines
Diet
Dipeptidyl Peptidase 4
Hep G2 Cells
In Vitro Techniques
Insulin
Interleukin-6
Kidney
Liver
Mesangial Cells
Metabolic Diseases
Mice
Mice, Obese
Obesity
RNA, Messenger
Sodium*
Blood Glucose
Butyric Acid
Cytokines
Dipeptidyl Peptidase 4
Insulin
Interleukin-6
RNA, Messenger
Sodium
Figure
Reference
-
1. Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998; 161:55–70. PMID: 9553764.
Article2. Choe EY, Cho Y, Choi Y, Yun Y, Wang HJ, Kwon O, Lee BW, Ahn CW, Cha BS, Lee HC, Kang ES. The effect of DPP-4 inhibitors on metabolic parameters in patients with type 2 diabetesd. Diabetes Metab J. 2014; 38:211–219. PMID: 25003075.3. Kim NH, Yu T, Lee DH. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors. Biomed Res Int. 2014; 2014:368703. PMID: 25140306.
Article4. Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, Mikami D, Needleman B, Satoskar AR, Rajagopalan S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013; 62:149–157. PMID: 22936179.
Article5. Lee SA, Kim YR, Yang EJ, Kwon EJ, Kim SH, Kang SH, Park DB, Oh BC, Kim J, Heo ST, Koh G, Lee DH. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:2553–2561. PMID: 23539735.
Article6. Ryskjaer J, Deacon CF, Carr RD, Krarup T, Madsbad S, Holst J, Vilsbøll T. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006; 155:485–493. PMID: 16914604.
Article7. Gu N, Tsuda M, Matsunaga T, Adachi T, Yasuda K, Ishihara A, Tsuda K. Glucose regulation of dipeptidyl peptidase IV gene expression is mediated by hepatocyte nuclear factor-1alpha in epithelial intestinal cells. Clin Exp Pharmacol Physiol. 2008; 35:1433–1439. PMID: 18671716.8. Böhm SK, Gum JR Jr, Erickson RH, Hicks JW, Kim YS. Human dipeptidyl peptidase IV gene promoter: tissue-specific regulation from a TATA-less GC-rich sequence characteristic of a housekeeping gene promoter. Biochem J. 1995; 311:835–843. PMID: 7487939.
Article9. Freeland KR, Wolever TM. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010; 103:460–466. PMID: 19818198.10. Lemoine M, Younes A. Histone deacetylase inhibitors in the treatment of lymphoma. Discov Med. 2010; 10:462–470. PMID: 21122478.11. Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009; 58:1509–1517. PMID: 19366864.
Article12. Jiang W, Guo Q, Wu J, Guo B, Wang Y, Zhao S, Lou H, Yu X, Mei X, Wu C, Qiao S, Wu Y. Dual effects of sodium butyrate on hepatocellular carcinoma cells. Mol Biol Rep. 2012; 39:6235–6242. PMID: 22228088.
Article13. Rodrigues MF, Carvalho É, Pezzuto P, Rumjanek FD, Amoêdo ND. Reciprocal modulation of histone deacetylase inhibitors sodium butyrate and trichostatin A on the energy metabolism of breast cancer cells. J Cell Biochem. 2015; 116:797–808. PMID: 25510910.
Article14. Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978; 14:105–113. PMID: 667927.
Article15. Ansorge S, Bank U, Heimburg A, Helmuth M, Koch G, Tadje J, Lendeckel U, Wolke C, Neubert K, Faust J, Fuchs P, Reinhold D, Thielitz A, Täger M. Recent insights into the role of dipeptidyl aminopeptidase IV (DPIV) and aminopeptidase N (APN) families in immune functions. Clin Chem Lab Med. 2009; 47:253–261. PMID: 19327105.
Article16. Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul Pept. 1999; 85:9–24. PMID: 10588446.
Article17. Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003; 40:209–294. PMID: 12892317.
Article18. Dang DT, Chun SY, Burkitt K, Abe M, Chen S, Havre P, Mabjeesh NJ, Heath EI, Vogelzang NJ, Cruz-Correa M, Blayney DW, Ensminger WD, St Croix B, Dang NH, Dang LH. Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition. Cancer Res. 2008; 68:1872–1880. PMID: 18339868.
Article19. Bauvois B, Djavaheri-Mergny M, Rouillard D, Dumont J, Wietzerbin J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene. 2000; 19:265–272. PMID: 10645005.
Article20. Cordero OJ, Salgado FJ, Viñuela JE, Nogueira M. Interleukin-12 enhances CD26 expression and dipeptidyl peptidase IV function on human activated lymphocytes. Immunobiology. 1997; 197:522–533. PMID: 9413751.21. Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006; 108:1602–1610. PMID: 16670267.
Article22. Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013; 12:125. PMID: 23984879.
Article23. Riemann D, Kehlen A, Langner J. Stimulation of the expression and the enzyme activity of aminopeptidase N/CD13 and dipeptidyl-peptidase IV/CD26 on human renal cell carcinoma cells and renal tubular epithelial cells by T cell-derived cytokines, such as IL-4 and IL-13. Clin Exp Immunol. 1995; 100:277–283. PMID: 7743667.
Article24. Stefanovic V, Ardaillou N, Vlahovic P, Placier S, Ronco P, Ardaillou R. Interferon-gamma induces dipeptidylpeptidase IV expression in human glomerular epithelial cells. Immunology. 1993; 80:465–470. PMID: 7904591.25. Tahara N, Yamagishi S, Takeuchi M, Tahara A, Kaifu K, Ueda S, Okuda S, Imaizumi T. Serum levels of advanced glycation end products (AGEs) are independently correlated with circulating levels of dipeptidyl peptidase-4 (DPP-4) in humans. Clin Biochem. 2013; 46:300–303. PMID: 23219738.
Article26. Yamabe T, Takakura K, Sugie K, Kitaoka Y, Takeda S, Okubo Y, Teshigawara K, Yodoi J, Hori T. Induction of the 2B9 antigen/dipeptidyl peptidase IV/CD26 on human natural killer cells by IL-2, IL-12 or IL-15. Immunology. 1997; 91:151–158. PMID: 9203979.
Article27. Boffa LC, Gruss RJ, Allfrey VG. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem. 1981; 256:9612–9621. PMID: 6270094.
Article28. Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond). 2015; 39:1331–1338. PMID: 25971927.
Article29. Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007; 5:981–989. PMID: 17951399.
Article30. Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013; 161:131–140. PMID: 23146568.
Article31. Ma X, Ezzeldin HH, Diasio RB. Histone deacetylase inhibitors: current status and overview of recent clinical trials. Drugs. 2009; 69:1911–1934. PMID: 19747008.32. Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008; 59(Suppl 2):251–262.33. Vidali G, Boffa LC, Bradbury EM, Allfrey VG. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978; 75:2239–2243. PMID: 276864.
Article34. Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011; 3:858–876. PMID: 22254083.
Article35. Malin SK, Huang H, Mulya A, Kashyap SR, Kirwan JP. Lower dipeptidyl peptidase-4 following exercise training plus weight loss is related to increased insulin sensitivity in adults with metabolic syndrome. Peptides. 2013; 47:142–147. PMID: 23872069.
Article36. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003; 133(7 Suppl):2485S–2493S. PMID: 12840228.
Article37. Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006; 21:351–366. PMID: 16870803.
Article38. den Besten G, Gerding A, van Dijk TH, Ciapaite J, Bleeker A, van Eunen K, Havinga R, Groen AK, Reijngoud DJ, Bakker BM. Protection against the metabolic syndrome by guar gum-derived short-chain fatty acids depends on peroxisome proliferator-activated receptor γ and glucagon-like peptide-1. PLoS One. 2015; 10:e0136364. PMID: 26292284.
Article39. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012; 61:364–371. PMID: 22190648.
Article40. Augustyns K, Bal G, Thonus G, Belyaev A, Zhang XM, Bollaert W, Lambeir AM, Durinx C, Goossens F, Haemers A. The unique properties of dipeptidyl-peptidase IV (DPP IV/CD26) and the therapeutic potential of DPP IV inhibitors. Curr Med Chem. 1999; 6:311–327. PMID: 10101215.41. Viollet B, Guigas B, Leclerc J, Hébrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 2009; 196:81–98. PMID: 19245656.
Article42. Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011; 93:891S–896S. PMID: 21325438.
Article43. Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012; 61:424–433. PMID: 21945106.
Article44. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007; 8:774–785. PMID: 17712357.
Article45. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010; 107:12553–12558. PMID: 20616029.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- Letter: Association between Serum Dipeptidyl Peptidase-4 Concentration and Obesity-Related Factors in Health Screen Examinees (J Obes Metab Syndr 2017;26:188-96)
- Comparison of DPP-4 Inhibitors
- Metabolic Consequences of Glucagon-Like Peptide-1 Receptor Agonist Shortage: Deterioration of Glycemic Control in Type 2 Diabetes