Cancer Res Treat.
2017 Jul;49(3):656-668. 10.4143/crt.2016.263.
Long Non-coding RNA HOXA11 Antisense Promotes Cell Proliferation and Invasion and Predicts Patient Prognosis in Serous Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, National Medical Center, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Yonsei University Graduate School, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea. ytkchoi@yuhs.ac
- KMID: 2388311
- DOI: http://doi.org/10.4143/crt.2016.263
Abstract
- PURPOSE
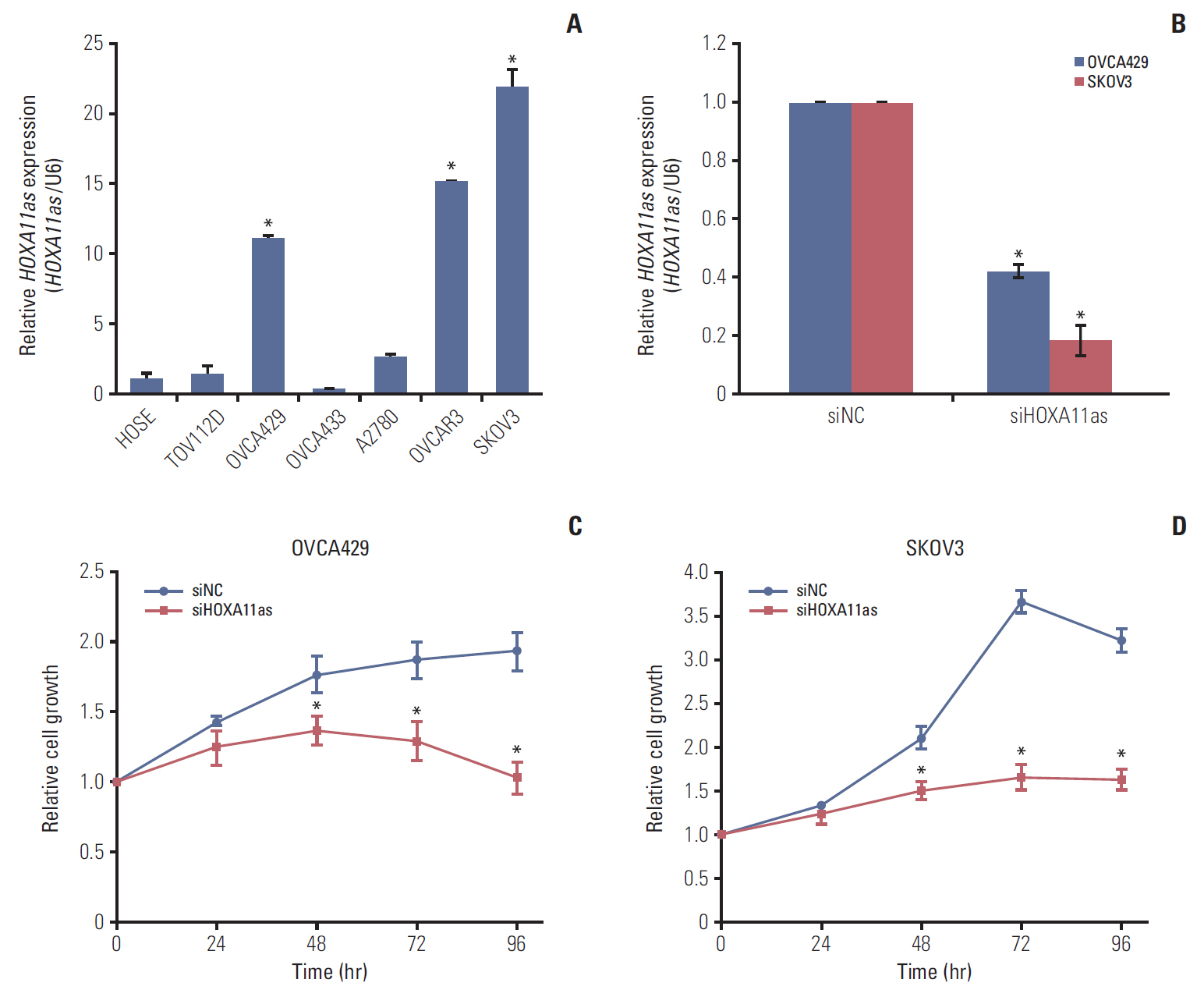
The biological function of long non-coding RNAs (lncRNAs) is only partially understood; therefore, in this study, we investigated the expression of the novel HOXA11 antisense (HOXA11as) lncRNA and its oncogenic role in serous ovarian cancer (SOC).
MATERIALS AND METHODS
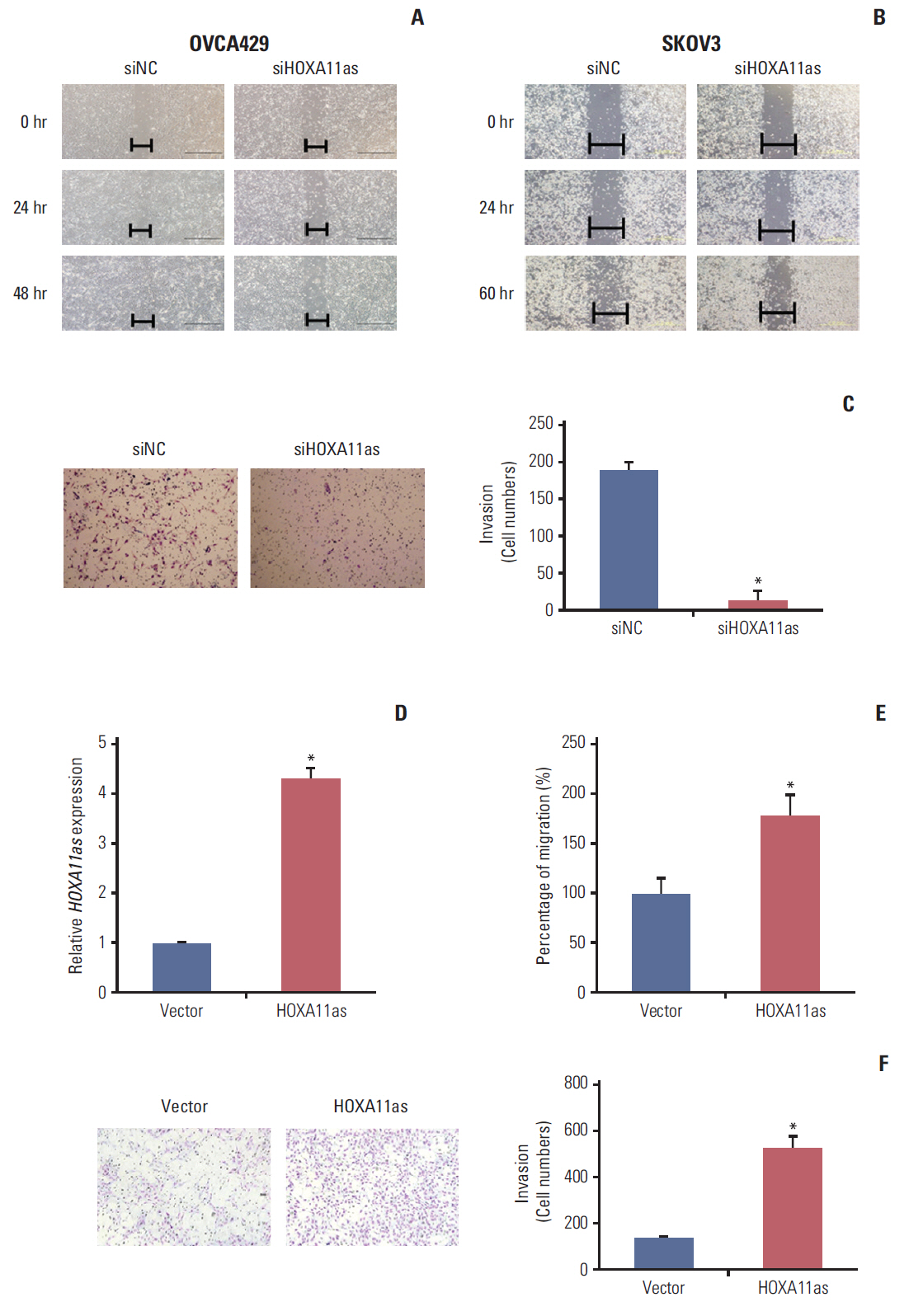
HOXA11as expression was examined in 129 SOC tissue samples by real time reverse transcription polymerase chain reaction. Clinicopathological factors and patient survival were compared between the high (n=27) and low HOXA11as expression group (n=102). To investigate the role of HOXA11as in cell proliferation, invasion, and migration, HOXA11as expression in ovarian cancer cells was knocked down using RNA interference.
RESULTS
HOXA11as expression in cancer tissue was 77-fold higher than that of noncancerous tissue (p < 0.05). Higher HOXA11as expression was significantly correlated with histological grade (p=0.017) and preoperative cancer antigen 125 (p=0.048). HOXA11as overexpression in SOC cells led to increased cell proliferation, invasion, and migration. Moreover, HOXA11as was associated with the expression of genes involved in cell invasion, migration, and epithelial-mesenchymal transition (EMT), including vascular endothelial growth factor, matrix metalloproteinase 9 (MMP-9), B-catenin, E-cadherin, Snail, Twist, and vimentin. Multivariate analysis revealed that HOXA11as was a prognostic factor of progressive disease and mortality (hazard ratio [HR], 1.730; p=0.043 and HR, 2.170; p=0.033, respectively). Progression-free and overall survival were significantly shorter in patients with high HOXA11as expression.
CONCLUSION
These findings highlight the clinical significance of HOXA11as to predicting the prognosis of SOC patients and suggest its potential in promoting tumor aggressiveness via regulation of vascular endothelial growth factor (VEGF), MMP-9, and EMT-related mechanisms.
MeSH Terms
-
Cadherins
Cell Proliferation*
Epithelial-Mesenchymal Transition
Humans
Matrix Metalloproteinase 9
Mortality
Multivariate Analysis
Ovarian Neoplasms*
Polymerase Chain Reaction
Prognosis*
Reverse Transcription
RNA Interference
RNA, Long Noncoding*
Snails
Vascular Endothelial Growth Factor A
Vimentin
Cadherins
Matrix Metalloproteinase 9
RNA, Long Noncoding
Vascular Endothelial Growth Factor A
Vimentin
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Menon U. Ovarian cancer: challenges of early detection. Nat Clin Pract Oncol. 2007; 4:498–9.
Article3. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014; 1839:1097–109.
Article4. Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009; 23:1494–504.
Article5. Chen T, Xie W, Xie L, Sun Y, Zhang Y, Shen Z, et al. Expression of long noncoding RNA lncRNA-n336928 is correlated with tumor stage and grade and overall survival in bladder cancer. Biochem Biophys Res Commun. 2015; 468:666–70.
Article6. Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013; 73:1180–9.
Article7. Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y, et al. Up-regulation of long noncoding RNA MALAT1 contributes to proliferation and metastasis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2015; 34:7.
Article8. Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med (Berl). 2014; 92:811–23.
Article9. Calvo R, West J, Franklin W, Erickson P, Bemis L, Li E, et al. Altered HOX and WNT7A expression in human lung cancer. Proc Natl Acad Sci U S A. 2000; 97:12776–81.
Article10. Cantile M, Cindolo L, Napodano G, Altieri V, Cillo C. Hyperexpression of locus C genes in the HOX network is strongly associated in vivo with human bladder transitional cell carcinomas. Oncogene. 2003; 22:6462–8.
Article11. Miller GJ, Miller HL, van Bokhoven A, Lambert JR, Werahera PN, Schirripa O, et al. Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res. 2003; 63:5879–88.12. Raman V, Martensen SA, Reisman D, Evron E, Odenwald WF, Jaffee E, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000; 405:974–8.
Article13. Vider BZ, Zimber A, Hirsch D, Estlein D, Chastre E, Prevot S, et al. Human colorectal carcinogenesis is associated with deregulation of homeobox gene expression. Biochem Biophys Res Commun. 1997; 232:742–8.
Article14. Garcia-Fernandez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005; 6:881–92.
Article15. Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005; 11:531–7.
Article16. Kelly ZL, Michael A, Butler-Manuel S, Pandha HS, Morgan RG. HOX genes in ovarian cancer. J Ovarian Res. 2011; 4:16.
Article17. Zhao M, Qiu Y, Yang B, Sun L, Hei K, Du X, et al. Long noncoding RNAs involved in gynecological cancer. Int J Gynecol Cancer. 2014; 24:1140–5.
Article18. Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim SW, et al. Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int J Oncol. 2015; 46:521–30.
Article19. Cheng Z, Guo J, Chen L, Luo N, Yang W, Qu X. A long noncoding RNA AB073614 promotes tumorigenesis and predicts poor prognosis in ovarian cancer. Oncotarget. 2015; 6:25381–9.
Article20. Gao Y, Meng H, Liu S, Hu J, Zhang Y, Jiao T, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015; 24:841–52.
Article21. Silva JM, Boczek NJ, Berres MW, Ma X, Smith DI. LSINCT5 is over expressed in breast and ovarian cancer and affects cellular proliferation. RNA Biol. 2011; 8:496–505.
Article22. Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S, et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015; 34:3076–84.
Article23. Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000; 36:1621–30.24. Geng YJ, Xie SL, Li Q, Ma J, Wang GY. Large intervening noncoding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011; 39:2119–28.
Article25. Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008; 19:294–308.
Article26. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006; 7:131–42.
Article27. Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013; 9:587–97.
Article28. Xu S, Sui S, Zhang J, Bai N, Shi Q, Zhang G, et al. Downregulation of long noncoding RNA MALAT1 induces epithelialto-mesenchymal transition via the PI3K-AKT pathway in breast cancer. Int J Clin Exp Pathol. 2015; 8:4881–91.29. Huang C, Yu W, Wang Q, Cui H, Wang Y, Zhang L, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med. 2015; 106:143–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Roles of Oncogenic Long Non-coding RNAs in Cancer Development
- DNM3OS Facilitates Ovarian Cancer Progression by Regulating miR-193a-3p/MAP3K3 Axis
- Differences of EDR Chemoresistance Assay and Prognosis between Recurrent Micropapillary Serous Ovarian Carcinoma and Serous Ovarian Carcinoma
- Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p
- What Is the Significance of Long Non-coding RNA HOX Transcript Antisense Intergenic RNA in Gastric Cancer?