Int J Stem Cells.
2015 Nov;8(2):181-190. 10.15283/ijsc.2015.8.2.181.
Comparative Histological Study on the Therapeutic Effect of Green Tea and Stem Cells in Alzheimer's Disease Complicating Experimentally Induced Diabetes
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Cairo University, Cairo, Egypt. maha_kaah@yahoo.com
- 2Department of Clinical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt.
- 3Department of General Surgery, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt.
- KMID: 2380812
- DOI: http://doi.org/10.15283/ijsc.2015.8.2.181
Abstract
- BACKGROUND AND OBJECTIVES
Alzheimer's disease (AD) is a devastating neurodegenerative disorder. Increasing evidence implicates diabetes mellitus (DM) as a risk factor for AD. Green tea (GT) has several beneficial effects attributed to its anti-oxidant phenolic compounds. Adipose tissue is a rich source of adipose-derived mesenchymal stem cells (ADSCs). This study was designed to evaluate and compare the possible therapeutic effect of green tea extract (GTE) and ADSCs on AD complicating induced DM in male rat.
METHODS
31 adult male albino rats were divided into 5 groups. Group I (Control), Group II received GTE, 50 mg/kg daily orally for 4 weeks, Group III received a single intraperitoneal injection of Streptozotocin (STZ), 50 mg/kg, Group IV: received STZ followed by GTE and Group V: received STZ followed by human ADSCs (hADSCs) intravenously.
RESULTS
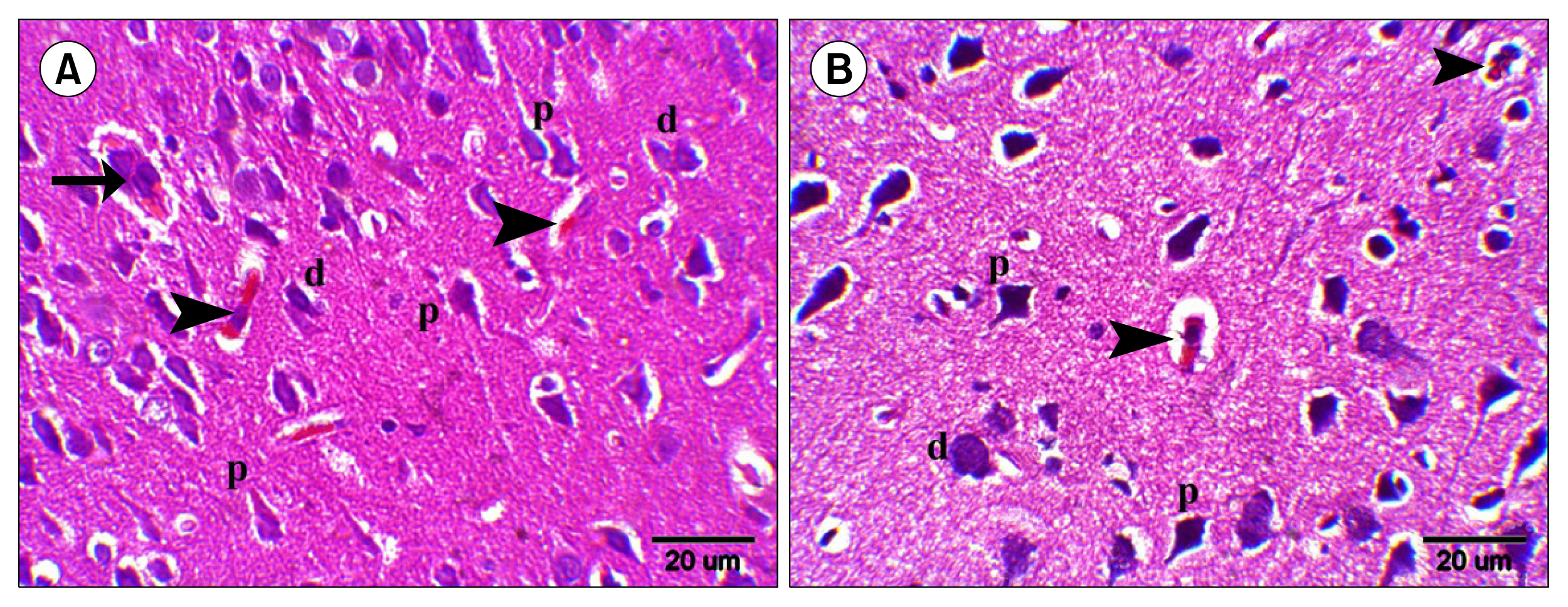
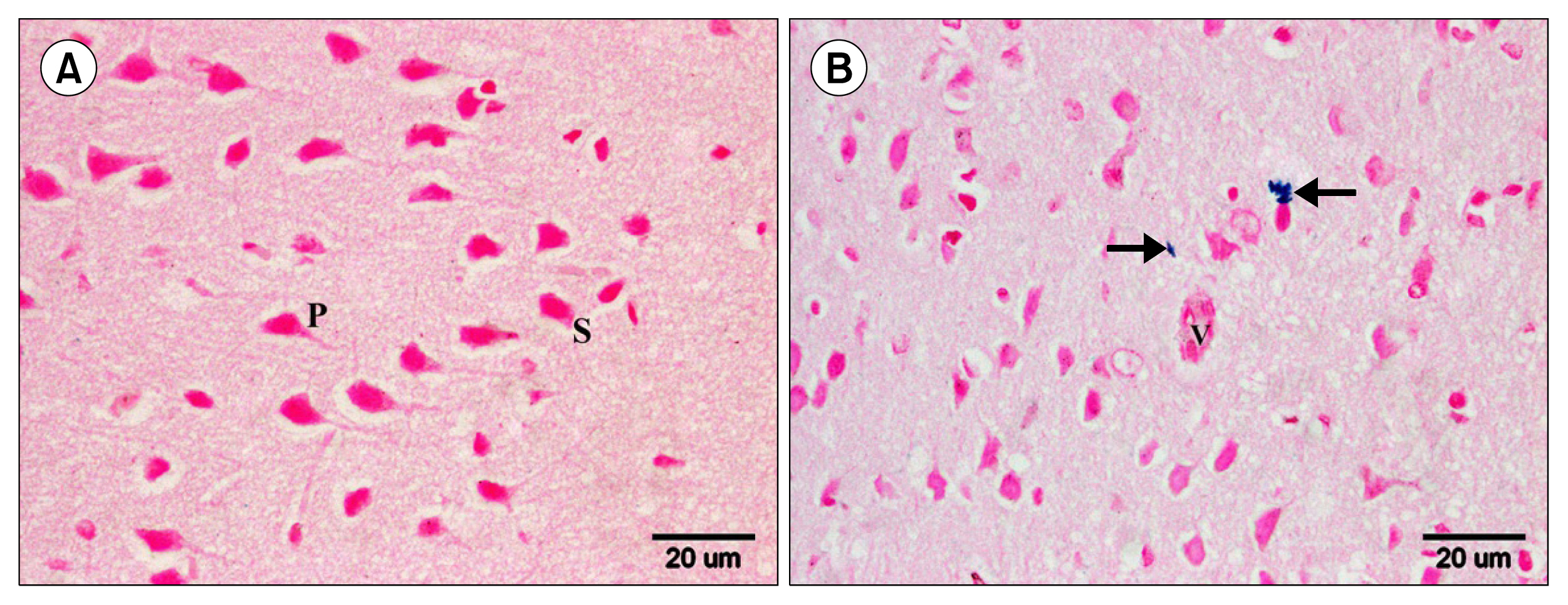
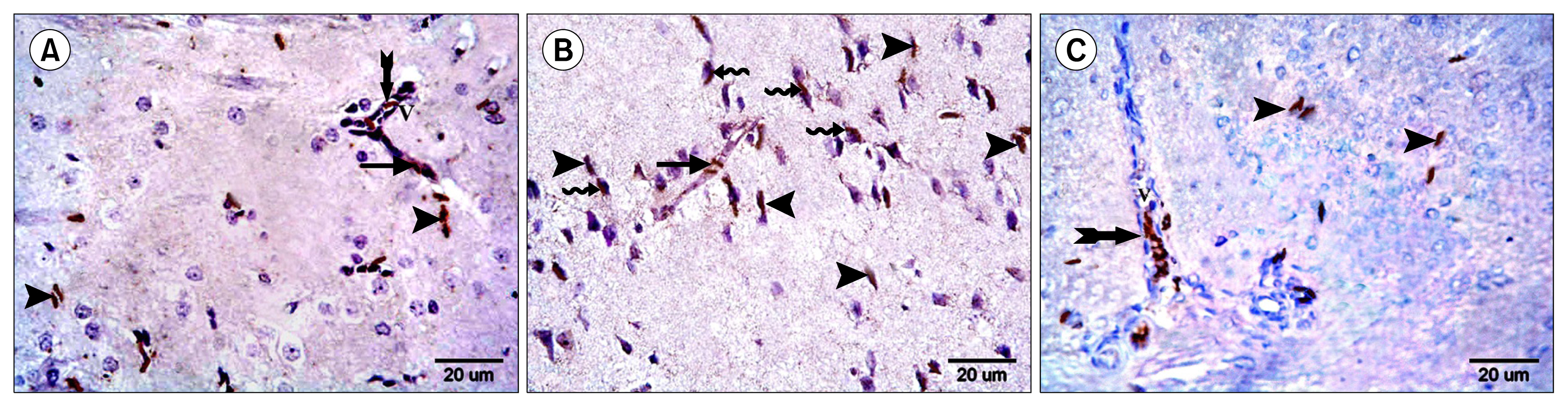
Multiple acidophilic masses, deformed neurons, Congo red +ve masses and Caspase 3 +ve neurons were seen in group III, became few in group IV and occasional in group V. Multiple Prussian blue +ve cells were detected in group V. Some CD44 +ve cells were noticed in group III, became multiple in groups IV and V. The mean area of neurons exhibiting acidophilic cytoplasm, mean area of amyloid plaques and mean area % of Caspase 3 +ve cells indicated a significant increase in group III. The mean area % of CD44 +ve cells recorded a significant increase in group IV.
CONCLUSIONS
hADSCs exerted a more marked therapeutic effect on the neurodegenerative changes complicating DM and corresponding to AD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Effect of Thymoquinone, α7 Receptor Agonist and α7 Receptor Allosteric Modulator on the Cerebral Cortex in Experimentally Induced Alzheimer’s Disease in Relation to MSCs Activation
Lamiaa Ibrahim AbdEl Fattah, Maha Baligh Zickri, Lobna Abdel Aal, Ola Heikal, Esraa Osama
Int J Stem Cells. 2016;9(2):230-238. doi: 10.15283/ijsc16021.
Reference
-
References
1. Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013; 108:21–43. DOI: 10.1016/j.pneurobio.2013.06.004. PMID: 23850509.
Article2. Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, Chiang CH, Huang PH, Chen TJ, Lin SJ, Chen JW, Chan WL. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PLoS One. 2014; 9:e87095–87101. DOI: 10.1371/journal.pone.0087095.
Article3. Wang JQ, Yin J, Song YF, Zhang L, Ren YX, Wang DG, Gao LP, Jing YH. Brain aging and AD-like pathology in streptozotocin-induced diabetic rats. J Diabetes Res. 2014; 2014:796840–796852. DOI: 10.1155/2014/796840. PMID: 25197672. PMCID: 4150474.
Article4. Namita P, Mukesh R, Vijay KJ. Camellia Sinensis (green tea): A review. Global Journal of Pharmacology. 2012; 6:52–59.5. Sykova E, Forostyak S. Stem cells in regenerative medicine. Laser Ther. 2013; 22:87–92. DOI: 10.5978/islsm.13-RE-01. PMID: 24155553. PMCID: 3806066.
Article6. Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011; 11:160–170. PMID: 21356171.7. Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013; 12:60–66. DOI: 10.1186/2251-6581-12-60. PMID: 24364898. PMCID: 3933000.
Article8. Cai Z, Yan Y, Wang Y. Minocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin Interv Aging. 2013; 8:1089–1095. DOI: 10.2147/CIA.S46536. PMID: 23983461. PMCID: 3749817.
Article9. Kim S, Chang KA, Kim Ja, Park HG, Ra JC, Kim HS, Suh YH. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS One. 2012; 7:e45757–45773. DOI: 10.1371/journal.pone.0045757.
Article10. Kakudo N, Morimoto N, Ogawa T, Kusumoto K. Potential of adipose-derived stem cells for regeneration medicine: clinical application and usefulness of fat grafting. J Stem Cell Res Ther. 2014; 4:1000204–1000206. DOI: 10.4172/2157-7633.1000204.
Article11. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007; 7:26–34. DOI: 10.1186/1472-6750-7-26. PMID: 17537254. PMCID: 1904213.
Article12. Haasters F, Prall WC, Anz D, Bourquin C, Pautke C, Endres S, Mutschler W, Docheva D, Schieker M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009; 214:759–767. DOI: 10.1111/j.1469-7580.2009.01065.x. PMID: 19438770. PMCID: 2707099.
Article13. Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003; 107:2290–2293. DOI: 10.1161/01.CIR.0000070931.62772.4E. PMID: 12732608.
Article14. Freshney RI. Culture of animal cells: A manual of basic technique. 3rd ed. New York: Wiley-Liss;1994. p. 105–148.15. Kiernan JA. Histological and histochemical methods : theory and practice. 3rd ed. London: Arnold;2001. p. 111–162.16. Wilcock DM, Gordon MN, Morgan D. Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat Protoc. 2006; 1:1591–1595. DOI: 10.1038/nprot.2006.277.
Article17. Ellis R. Perls Prussian blue Stain Protocol. IMVS division of pathology, The queen elizabeth hospital;South Australia: 2007.18. Bancroft JD, Cook HC. Immunocytochemistry. Manual of histological techniques and their diagnostic applications. 2nd ed. Edinburgh: Churchill Livingstone;1994. p. 263–325.19. Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke. 2013; 44:551–554. DOI: 10.1161/STROKEAHA.112.676759.
Article20. Wang Q, Zhou L, Guo Y, Liu G, Cheng J, Yu H. Differentiation of human adipose-derived stem cells into neuron-like cells by Radix Angelicae Sinensis. Neural Regen Res. 2013; 8:3353–3358.21. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010; 19:237–270. DOI: 10.1177/0962280209105014.
Article22. Liu J, Zhang Y, Deng X, Yin F. Geniposide decreases the level of Aβ1-42 in the hippocampus of streptozotocin-induced diabetic rats. Acta Biochim Biophys Sin (Shanghai). 2013; 45:787–791. DOI: 10.1093/abbs/gmt069.
Article23. Girard SD, Baranger K, Gauthier C, Jacquet M, Bernard A, Escoffier G, Marchetti E, Khrestchatisky M, Rivera S, Roman FS. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2013; 33:781–796.
Article24. Garman RH. Histology of the central nervous system. Toxicol Pathol. 2011; 39:22–35. DOI: 10.1177/0192623310389621.
Article25. Palmer AM. Neuroprotective therapeutics for Alzheimer’s disease: progress and prospects. Trends Pharmacol Sci. 2011; 32:141–147. DOI: 10.1016/j.tips.2010.12.007. PMID: 21256602.
Article26. Luebke JI, Weaver CM, Rocher AB, Rodriguez A, Crimins JL, Dickstein DL, Wearne SL, Hof PR. Dendritic vulnerability in neurodegenerative disease: insights from analyses of cortical pyramidal neurons in transgenic mouse models. Brain Struct Funct. 2010; 214:181–199. DOI: 10.1007/s00429-010-0244-2. PMID: 20177698. PMCID: 3045830.
Article27. Chabrier MA, Cheng D, Castello NA, Green KN, LaFerla FM. Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer’s disease. Neurobiol Dis. 2014; 64:107–117. DOI: 10.1016/j.nbd.2014.01.007. PMID: 24440055. PMCID: 4072239.
Article28. Bonda DJ, Wang X, Lee HG, Smith MA, Perry G, Zhu X. Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci Bull. 2014; 30:243–252. DOI: 10.1007/s12264-013-1424-x. PMID: 24733654. PMCID: 4097013.
Article29. Salem AM, Sabry GM, Ahmed HH, Hussein AA, Kotob SE. Amelioration of neuroinflammation and apoptosis characterizing Alzheimer’s disease by natural products. Int J Pharm Pharm Sci. 2013; 5(2 Suppl):87–94.30. Lee ST, Chu K, Park JE, Jung KH, Jeon D, Lim JY, Lee SK, Kim M, Roh JK. Erythropoietin improves memory function with reducing endothelial dysfunction and amyloid-beta burden in Alzheimer’s disease models. J Neurochem. 2012; 120:115–124. DOI: 10.1111/j.1471-4159.2011.07534.x.
Article31. Kovacs GG. Practical approach to diagnosis: sampling and basic stainings. Kovacs GG, editor. Neuropathology of neurodegenerative diseases: A practical guide. United Kingdom: Cambridge University Press;2015. p. 55–69.32. Khairallah MI, Kassem LA, Yassin NA, El Din MA, Zekri M, Attia M. The hematopoietic growth factor “erythropoietin” enhances the therapeutic effect of mesenchymal stem cells in Alzheimer’s disease. Pak J Biol Sci. 2014; 17:9–21. DOI: 10.3923/pjbs.2014.9.21. PMID: 24783773.
Article33. Castellani RJ, Perry G. The complexities of the pathology-pathogenesis relationship in Alzheimer disease. Biochem Pharmacol. 2014; 88:671–676. DOI: 10.1016/j.bcp.2014.01.009. PMID: 24447936.
Article34. Bhaskar K, Maphis N, Xu G, Varvel NH, Kokiko-Cochran ON, Weick JP, Staugaitis SM, Cardona A, Ransohoff RM, Herrup K, Lamb BT. Microglial derived tumor necrosis factor-α drives Alzheimer’s disease-related neuronal cell cycle events. Neurobiol Dis. 2014; 62:273–285. DOI: 10.1016/j.nbd.2013.10.007.
Article35. Noguchi-Shinohara M, Yuki S, Dohmoto C, Ikeda Y, Samuraki M, Iwasa K, Yokogawa M, Asai K, Komai K, Nakamura H, Yamada M. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014; 9:e96013–96020. DOI: 10.1371/journal.pone.0096013. PMID: 24828424. PMCID: 4020750.
Article36. Choi SS, Lee SR, Kim SU, Lee HJ. Alzheimer’s disease and stem cell therapy. Exp Neurobiol. 2014; 23:45–52. DOI: 10.5607/en.2014.23.1.45. PMID: 24737939. PMCID: 3984956.
Article37. Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014; 21:216–225. DOI: 10.1038/cdd.2013.158. PMCID: 3890955.
Article38. Zickri MB, Embaby A. Relation between endogenous stem cells and green tea extract in overconsumption and amiodarone induced thyroid damage in rat. Int J Stem Cells. 2013; 6:113–120. DOI: 10.15283/ijsc.2013.6.2.113.
Article39. Salem AM, Ahmed HH, Atta HM, Ghazy MA, Aglan HA. Potential of bone marrow mesenchymal stem cells in management of Alzheimer’s disease in female rats. Cell Biol Int. 2014; 38:1367–1383. DOI: 10.1002/cbin.10331. PMID: 25044885.
Article40. Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012; 30:804–810. DOI: 10.1002/stem.1076. PMID: 22415904.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- The Effects of Green Tea on Obesity and Type 2 Diabetes
- Effect of Green Tea on Calcium Oxalate Stone Formation and Excretion in Ethylene Glycol-treated Rats
- Relation between Endogenous Stem Cells and Green Tea Extract in Overconsumption and Amiodarone Induced Thyroid Damage in Rat
- A Case of Green Tea-induced Occupational Asthma