J Vet Sci.
2015 Sep;16(3):307-315. 10.4142/jvs.2015.16.3.307.
Quantitative determination of 12-hydroxyeicosatetraenoic acids by chiral liquid chromatography tandem mass spectrometry in a murine atopic dermatitis model
- Affiliations
-
- 1Laboratory of Toxicology, BK21 PLUS Program for Creative Veterinary Science Research, Research Institute for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. dmdgh82@naver.com, mchotox@snu.ac.kr
- 2Chemical and Material Components Center, Korea Testing Certification (KTC), Gunpo 435-823, Korea.
- 3Department of Clinical Pathology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 4Graduate School of Convergence Science and Technology, Seoul National University, Suwon 443-270, Korea.
- 5Graduate Group of Tumor Biology, Seoul National University, Seoul 151-742, Korea.
- 6Advanced Institute of Convergence Technology, Seoul National University, Suwon 443-270, Korea.
- 7Institute of Green Bio Science Technology, Seoul National University, Pyeongchang 232-916, Korea.
- KMID: 2344303
- DOI: http://doi.org/10.4142/jvs.2015.16.3.307
Abstract
- Atopic dermatitis, one of the most important skin diseases, is characterized by both skin barrier impairment and immunological abnormalities. Although several studies have demonstrated the significant relationship between atopic dermatitis and immunological abnormalities, the role of hydroxyeicosatetraenoic acids (HETE) in atopic dermatitis remains unknown. To develop chiral methods for characterization of 12-HETE enantiomers in a 1-chloro-2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mouse model and evaluate the effects of 12-HETE on atopic dermatitis, BALB/c mice were treated with either DNCB or acetone/olive oil (AOO) to induce atopic dermatitis, after which 12(R)- and 12(S)-HETEs in the plasma, skin, spleen, and lymph nodes were quantified by chiral liquid chromatography-tandem mass spectrometry. 12(R)- and 12(S)-HETEs in biological samples of DNCB-induced atopic dermatitis mice increased significantly compared with the AOO group, reflecting the involvement of 12(R)- and 12(S)-HETEs in atopic dermatitis. These findings indicate that 12(R)- and 12(S)-HETEs could be a useful guide for understanding the pathogenesis of atopic dermatitis.
Keyword
MeSH Terms
-
Animals
Biomarkers/blood/metabolism
*Chromatography, Liquid
Dermatitis, Atopic/*chemically induced
Dinitrochlorobenzene/adverse effects
Female
Humans
Hydroxyeicosatetraenoic Acids/blood/*metabolism
Irritants/adverse effects
Mice
Mice, Inbred BALB C
Models, Animal
*Tandem Mass Spectrometry
Biomarkers
Dinitrochlorobenzene
Hydroxyeicosatetraenoic Acids
Irritants
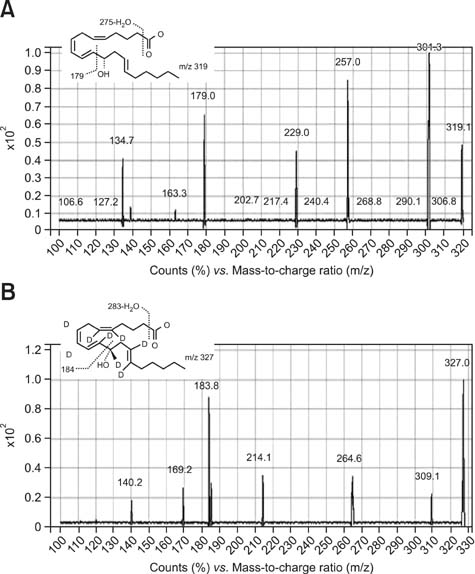
Figure
Reference
-
1. Bayer M, Mosandl A, Thaçi D. Improved enantioselective analysis of polyunsaturated hydroxy fatty acids in psoriatic skin scales using high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 819:323–328.
Article2. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959; 37:911–917.
Article3. De Benedetto A, Agnihothri R, McGirt LY, Bankova LG, Beck LA. Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol. 2009; 129:14–30.
Article4. de Grauw JC, van de Lest CHA, van Weeren PR. A targeted lipidomics approach to the study of eicosanoid release in synovial joints. Arthritis Res Ther. 2011; 13:R123.
Article5. Dorsh W. Late Phase Allergic Reactions. Boca Raton: CRC Press;1990.6. Epp N, Fürstenberger G, Müller K, de Juanes S, Leitges M, Hausser I, Thieme F, Liebisch G, Schmitz G, Krieg P. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J Cell Biol. 2007; 177:173–182.
Article7. Fogh K, Herlin T, Kragballe K. Eicosanoids in skin of patients with atopic dermatitis: prostaglandin E2 and leukotriene B4 are present in biologically active concentrations. J Allergy Clin Immunol. 1989; 83(Pt 1):450–455.
Article8. Hammarström S, Lindgren JA, Marcelo C, Duell EA, Anderson TF, Voorhees JJ. Arachidonic acid transformations in normal and psoriatic skin. J Invest Dermatol. 1979; 73:180–183.
Article9. Lee JK, Shin JS, Kim JH, Eom JH, Son KH, Kil JH, Kim JR, Yoon BI, Kim HS, Park KL. Evaluation of immunosafety for skin sensitization induced by chemicals. National Institute of Toxicological Research. The Annual Report of Korea National Toxicology Program 2005. Vol. 4. Seoul: National Institute of Toxicological Research;2006. p. 81–90.10. Lee SH, Williams MV, DuBois RN, Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom. 2003; 17:2168–2176.
Article11. McDonnell M, Davis W Jr, Li H, Funk CD. Characterization of the murine epidermal 12/15-lipoxygenase. Prostaglandins Other Lipid Mediat. 2001; 63:93–107.
Article12. Neuber K, Hilger RA, König W. Differential increase in 12-HETE release and CD29/CD49f expression of platelets from normal donors and from patients with atopic dermatitis by Staphylococcus aureus. Int Arch Allergy Immunol. 1992; 98:339–342.
Article13. Nicolaou A. Eicosanoids in skin inflammation. Prostaglandins Leukot Essent Fatty Acids. 2013; 88:131–138.
Article14. Ozdamar SO, Seçkin D, Kandemir B, Turanli AY. Mast cells in psoriasis. Dermatology. 1996; 192:190.
Article15. Park SJ, Lee HA, Kim JW, Lee BS, Kim EJ. Platycodon grandiflorus alleviates DNCB-induced atopy-like dermatitis in NC/Nga mice. Indian J Pharmacol. 2012; 44:469–474.
Article16. Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. NC/Nga mice: a mouse model for atopicdermatitis. Int Arch Allergy Immunol. 1999; 120:Suppl 1. 70–75.17. Yamamoto M, Haruna T, Yasui K, Takahashi H, Iduhara M, Takaki S, Deguchi M, Arimura A. A novel atopic dermatitis model induced by topical application with Dermatophagoides farinae extract in NC/Nga mice. Allergol Int. 2007; 56:139–148.
Article18. Yamamoto S, Suzuki H, Ueda N, Takahashi Y, Yoshimoto T. Mammalian lipoxygenases. In : Curtis-Prior PB, editor. The Eicosanoids. Chichester: John Wiley & Sons;2004. p. 53–59.19. Zheng Y, Yin H, Boeglin WE, Elias PM, Crumrine D, Beier DR, Brash AR. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: a proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. J Biol Chem. 2011; 286:24046–24056.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation Between Serum Vitamin D Level and the Severity of Atopic Dermatitis Associated With Food Sensitization
- Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits
- Qualitative and Quantitative Analysis of Thirteen Marker Components in Traditional Korean Formula, Samryeongbaekchul-san using an Ultra-Performance Liquid Chromatography Equipped with Electrospray Ionization Tandem Mass Spectrometry
- Determination of Phthalate Metabolites in Human Serum and Urine as Biomarkers for Phthalate Exposure Using Column-Switching LC-MS/MS
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry