Transl Clin Pharmacol.
2017 Dec;25(4):183-189. 10.12793/tcp.2017.25.4.183.
Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. yimds@catholic.ac.kr
- 2Department of Biomedical Science, BK21 Plus KNU Bio-Medical Convergence Program for Creative Talent and Clinical Trial Center, Kyungpook National University Graduate School and Hospital, Daegu 41944, Korea.
- KMID: 2407000
- DOI: http://doi.org/10.12793/tcp.2017.25.4.183
Abstract
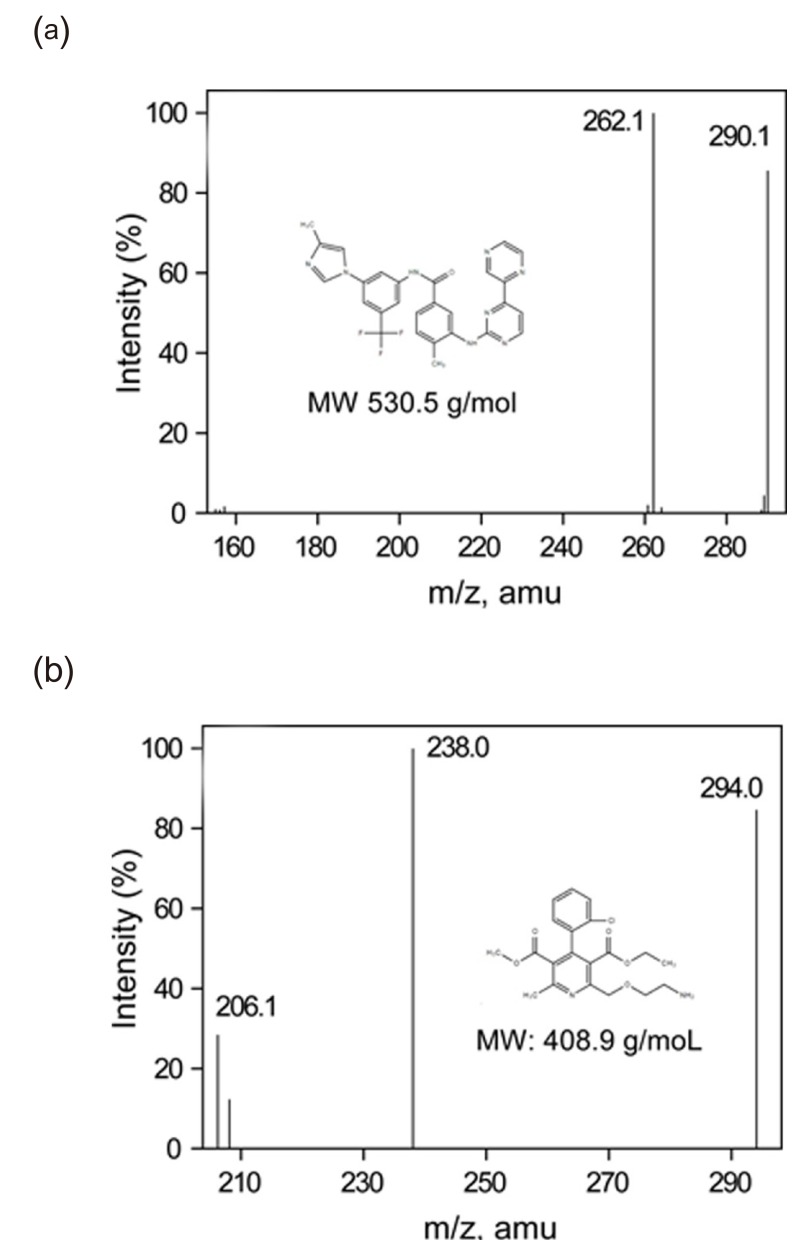
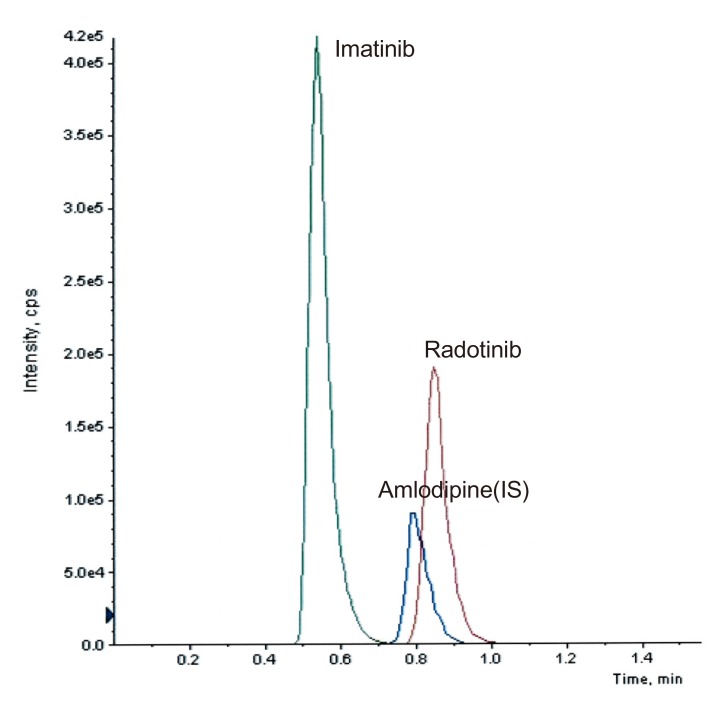
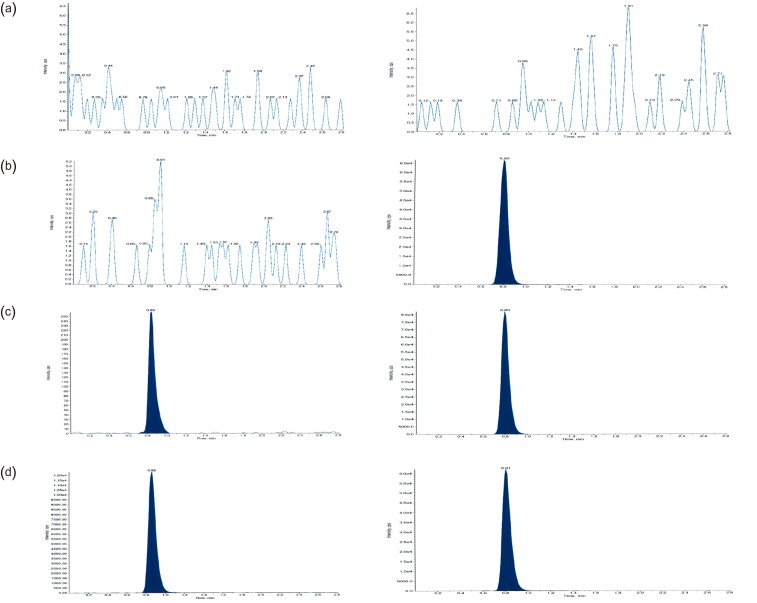
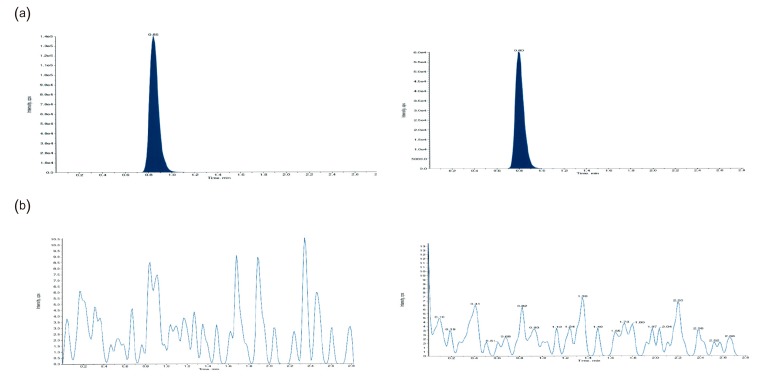
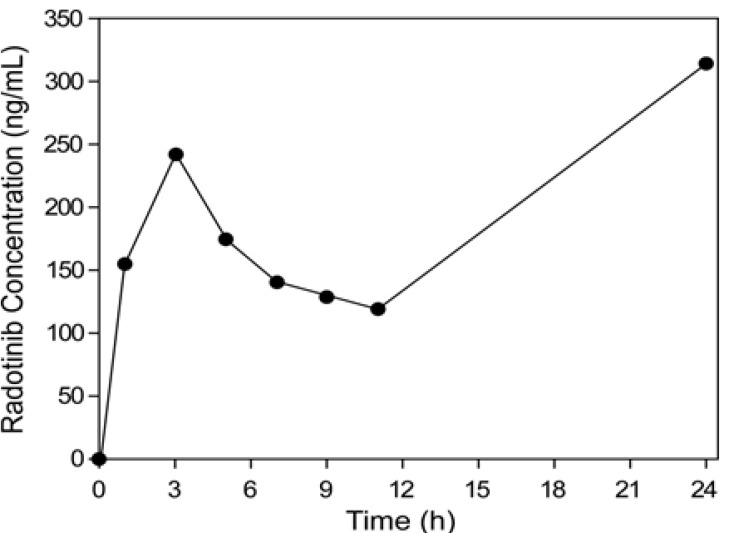
- This study describes the development of an analytical method to determine radotinib levels in human plasma using high performance liquid chromatography (HPLC) coupled with triple quadrupole tandem mass spectrometry (MS/MS) for pharmacokinetic application. Plasma samples were sequentially processed by liquid-liquid extraction using methyl tert-butyl ether, evaporation, and reconstitution. Analytes were separated and analyzed using HPLC-MS/MS in selected reaction monitoring mode, monitoring the specific transitions of m/z 531 to 290 for radotinib and m/z 409 to 238 for amlodipine (internal standard). The HPLC-MS/MS analytical method was validated with respect to selectivity, linearity, sensitivity, accuracy, precision, recovery, and stability. Calibration curves were linear over a concentration range 5-3,000 ng/mL with correlation coefficients (r) > 0.998. The lower limit of quantification for radotinib in plasma was 5 ng/mL. The accuracy and precision of the analytical method were acceptable within 15% at all quality control levels. This method was suitable to determine radotinib levels in human plasma because of its simplicity, selectivity, precision, and accuracy.
MeSH Terms
Figure
Reference
-
1. Kim SH, Menon H, Jootar S, Saikia T, Kwak JY, Sohn SK, et al. Efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors. Haematologica. 2014; 99:1191–1196. DOI: 10.3324/haematol.2013.096776. PMID: 24705186.
Article2. Eskazan AE, Soysal T. Radotinib in the treatment of chronic phase chronic myeloid leukemia patients. Haematologica. 2015; 100:e39. DOI: 10.3324/haematol.2014.117846. PMID: 25552681.
Article3. Yun S, Vincelette ND, Segar JM, Dong Y, Shen Y, Kim DW, et al. Comparative Effectiveness of Newer Tyrosine Kinase Inhibitors Versus Imatinib in the First-Line Treatment of Chronic-Phase Chronic Myeloid Leukemia Across Risk Groups: A Systematic Review and Meta-Analysis of Eight Randomized Trials. Clin Lymphoma Myeloma Leuk. 2016; 16:e85–e94. DOI: 10.1016/j.clml.2016.03.003. PMID: 27101984.
Article4. Zabriskie MS, Vellore NA, Gantz KC, Deininger MW, O'Hare T. Radotinib is an Effective Inhibitor of Native and Kinase Domain-Mutant BCR-ABL1. Leukemia. 2015; 29:1939–1942. DOI: 10.1038/leu.2015.42. PMID: 25676420.
Article5. US Food and Drug Administration. Guidance for Industry: Bioanalytical method validation. Accessed 24 October 2017. https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.6. MFDS. Guideline for the validation of bioanalytical method. Accessed 24 October 2017. http://drug.mfds.go.kr/Data/KO_DU/20100804092704_0.hwp.7. Bylda C, Thiele R, Kobold U, Volmer DA. Recent advances in sample preparation techniques to overcome difficulties encountered during quantitative analysis of small molecules from biofluids using LC-MS/MS. Analyst. 2014; 139:2265–2276. DOI: 10.1039/c4an00094c. PMID: 24633191.
Article8. Hughes NC, Wong EY, Fan J, Bajaj N. Determination of carryover and contamination for mass spectrometry-based chromatographic assays. AAPS J. 2007; 9:e353–e360. DOI: 10.1208/aapsj0903042. PMID: 18170982.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Novel Simultaneous Determination of Sarpogrelate and its Active Metabolite (M-1) in Human Plasma, Using Liquid Chromatography-Tandem Mass Spectrometry: Clinical Application
- Determination of sumatriptan in human plasma using liquid chromatography-mass spectrometry for pharmacokinetic study in healthy Korean volunteers
- A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid
- Determination of donepezil in human plasma using ultra performance liquid chromatography-tandem mass spectrometry
- Quantification of apixaban in human plasma using ultra performance liquid chromatography coupled with tandem mass spectrometry