J Menopausal Med.
2014 Apr;20(1):21-31.
Mitochondrial Channel Opener Diazoxide Attenuates Hypoxia-Induced sFlt-1 Release in Human Choriocarcinoma Cells
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Pusan National University School of Medicine, Busan, Korea. bislsan@naver.com
Abstract
OBJECTIVES
To examine the effect of diazoxide on hypoxia-induced soluble fms-like tyrosin kinase-1 (sFlt-1) release in JEG-3 choriocarcinoma cells.
METHODS
Cells were cultured under normoxia (20% O2) or hypoxia (1% O2), and expression of sFlt-1 mRNA and protein release was determined by quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) assays and enzyme-linked immunosorbent assay (ELISA).
RESULTS
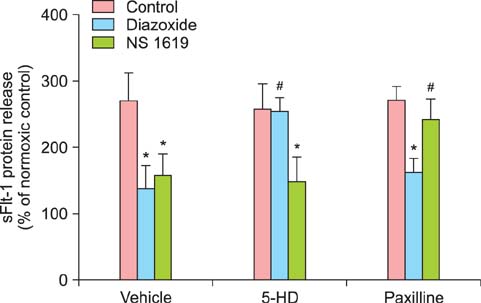
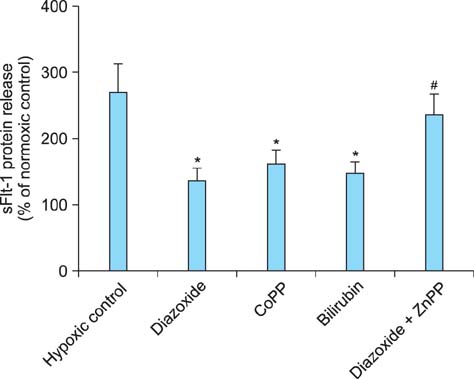
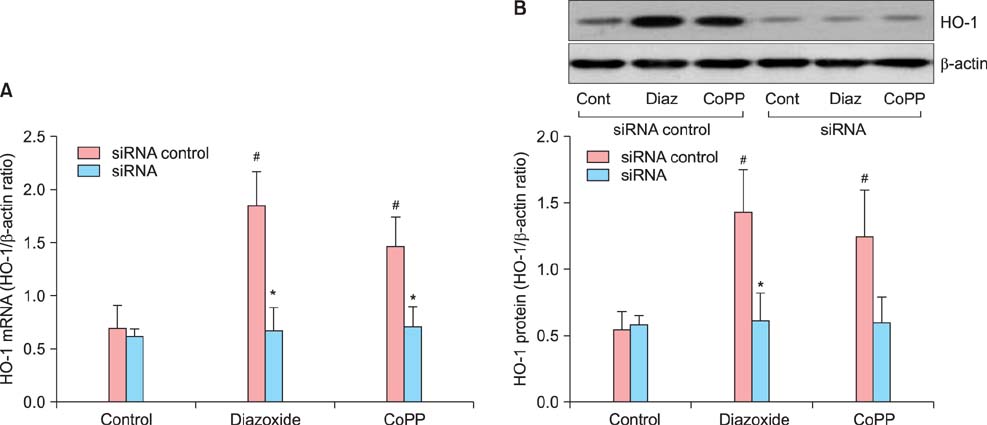
Tumor necrosis factor-alpha (TNF-alpha) as well as hypoxia stimulated sFlt-1 release and diazoxide inhibited both of them. The selective inhibitor of mitochondrial adenosine triphosphat (ATP)-sensitive K+ channel opener (K(ATP)) 5-hydroxydecanoate (5-HD) completely reversed the diazoxide-induced inhibition of hypoxia-stimulated sFlt-1 release. qRT-PCR and Western blot analyses showed that diazoxide up-regulated the heme oxygenase-1 (HO-1) expression. In addition, the HO-1 inducer cobalt protoporphyrin (CoPP) and the metabolic product of HO-1 bilirubin mimicked diazoxide to inhibit sFlt-1 release and reactive oxygen species (ROS) production under hypoxia, whereas the HO-1 inhibitor zinc protoporphyrin IX (ZnPP IX) antagonized the effect of diazoxide. In cells transfected with the HO-1 siRNA, diazoxide did not exert any effect on sFlt-1 release and ROS production under hypoxia.
CONCLUSION
These results, taken together, strongly suggest that up-regulation of the HO-1 expression is the crucial mechanism responsible for the diazoxide-induced inhibition of the sFlt-1 release and ROS production under hypoxia.
Keyword
MeSH Terms
-
Adenosine
Anoxia
Bilirubin
Blotting, Western
Choriocarcinoma*
Cobalt
Diazoxide*
Enzyme-Linked Immunosorbent Assay
Female
Heme Oxygenase-1
Humans
Polymerase Chain Reaction
Pregnancy
Reactive Oxygen Species
RNA, Messenger
RNA, Small Interfering
Tumor Necrosis Factor-alpha
Up-Regulation
Vascular Endothelial Growth Factor Receptor-1
Zinc
Adenosine
Bilirubin
Cobalt
Diazoxide
Heme Oxygenase-1
RNA, Messenger
RNA, Small Interfering
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor Receptor-1
Zinc
Figure
Reference
-
1. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995; 146:1029–1039.2. de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992; 255:989–991.3. Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992; 187:1579–1586.4. Crescimanno C, Marzioni D, Persico MG, Vuckovic M, Mühlhauser J, Castellucci M. Expression of bFGF, PIGF and their receptors in the human placenta. Placenta. 1995; 16:A13.5. Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod. 1996; 11:1090–1098.6. Vuckovic M, Ponting J, Terman BI, Niketic V, Seif MW, Kumar S. Expression of the vascular endothelial growth factor receptor, KDR, in human placenta. J Anat. 1996; 188(Pt 2):361–366.7. Charnock-Jones DS, Sharkey AM, Boocock CA, Ahmed A, Plevin R, Ferrara N, et al. Vascular endothelial growth factor receptor localization and activation in human trophoblast and choriocarcinoma cells. Biol Reprod. 1994; 51:524–530.8. Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors. 1995; 12:235–243.9. Villars DS. The equilibrium constants of reactions involving hydroxyl. Proc Natl Acad Sci U S A. 1929; 15:705–709.10. Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996; 226:324–328.11. Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, et al. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998; 59:1540–1548.12. Anthony FW, Evans PW, Wheeler T, Wood PJ. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Ann Clin Biochem. 1997; 34(Pt 3):276–280.13. Ahmad S, Ahmed A. Regulation of soluble VEGFR-1 by VEGF and oxygen and its elevation in pre-eclampsia and fetal growth restriction. Placenta. 2001; 22:A7.14. Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmäki E. Amniotic fluid--soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol. 2000; 95:353–357.15. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111:649–658.16. Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004; 95:884–891.17. Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010; 14:1857–1867.18. Kersten JR, Gross GJ, Pagel PS, Warltier DC. Activation of adenosine triphosphate-regulated potassium channels: mediation of cellular and organ protection. Anesthesiology. 1998; 88:495–513.19. Szewczyk A, Marbán E. Mitochondria: a new target for K channel openers? Trends Pharmacol Sci. 1999; 20:157–161.20. Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, et al. Mechanisms by which opening the mitochondrial ATP-sensitive K(+) channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002; 283:H284–H295.21. Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, et al. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol. 2002; 97:365–373.22. Ferranti R, da Silva MM, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channel opening decreases reactive oxygen species generation. FEBS Lett. 2003; 536:51–55.23. Xu B, Makris A, Thornton C, Ogle R, Horvath JS, Hennessy A. Antihypertensive drugs clonidine, diazoxide, hydralazine and furosemide regulate the production of cytokines by placentas and peripheral blood mononuclear cells in normal pregnancy. J Hypertens. 2006; 24:915–922.24. Zeng Z, Huang HF, He F, Wu LX, Lin J, Chen MQ. Diazoxide attenuates ischemia/reperfusion injury via upregulation of heme oxygenase-1 after liver transplantation in rats. World J Gastroenterol. 2012; 18:1765–1772.25. Redman CW, Sargent IL. Placental stress and preeclampsia: a revised view. Placenta. 2009; 30:Suppl A. S38–S42.26. Schmidt HHHW, Kelm M. Determination of nitrite and nitrate by the Griess reaction. In : Feelisch M, Stamler JS, editors. Methods in nitric oxide research. Chichester: John Wiley & Sons;1996. p. 491–497.27. Robertson DW, Steinberg MI. Potassium channel modulators: scientific applications and therapeutic promise. J Med Chem. 1990; 33:1529–1541.28. Marks GS. Heme oxygenase: the physiological role of one of its metabolites, carbon monoxide and interactions with zinc protoporphyrin, cobalt protoporphyrin and other metalloporphyrins. Cell Mol Biol (Noisy-le-grand). 1994; 40:863–870.29. Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B, et al. Resveratrol inhibits the release of soluble fmslike tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012; 206:253.e10–253.e15.30. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007; 335:974.31. Drose S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006; 281:23733–23739.32. Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, et al. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003; 284:H1048–H1056.33. Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004; 109:1714–1717.34. Cao J, Inoue K, Li X, Drummond G, Abraham NG. Physiological significance of heme oxygenase in hypertension. Int J Biochem Cell Biol. 2009; 41:1025–1033.35. Botros FT, Schwartzman ML, Stier CT Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005; 68:2745–2755.36. Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001; 38:210–215.37. Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension. 2004; 43:1221–1226.38. Neuzil J, Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Lett. 1993; 331:281–284.39. Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994; 269:16712–16719.40. Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005; 57:585–630.41. Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007; 115:1789–1797.42. Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995; 96:2676–2682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sildenafil Inhibits Advanced Glycation End Products-induced sFlt-1 Release Through Upregulation of Heme Oxygenase-1
- Effects of Diaxoxide, Cromakalim and Pinacidil on Acetylcholine and Norepinephrine Release in Rat Hippocampus
- Comparative Studies of KATP Channel Openers in Penile Erection: Minoxidil, Diazoxide and Pinacidil
- Differential Changes of ATP-sensitive Potassium Channel Current after Hypoxia-reperfusion Treatment in Mouse Neuroblastoma 2a (N2a) Cell
- The Role of Mitochondrial ATP-sensitive Potassium Channel on Intestinal Pacemaking Activity