World J Mens Health.
2014 Dec;32(3):123-132. 10.5534/wjmh.2014.32.3.123.
Understanding the Role of Heat Shock Protein Isoforms in Male Fertility, Aging and Apoptosis
- Affiliations
-
- 1Institute of Science, Nirma University, Gujarat, India. sriram.seshadri@nirmauni.ac.in
- KMID: 2320775
- DOI: http://doi.org/10.5534/wjmh.2014.32.3.123
Abstract
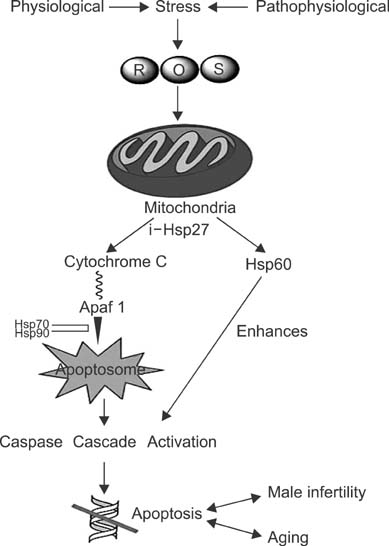
- Heat shock proteins (HSPs) play a role in the homeostasis, apoptosis regulation and the maintenance of the various other physiological processes. Aging is accompanied by a decrease in the resistance to environmental stress, while mitochondria are primary targets in the process of aging, their expression decreasing with age. Mitochondrion also plays a significant role in the process of spermatogenesis. HSPs have been shown to be involved in apoptosis with some of acting as apoptotic inhibitors and are involved in cytoprotection. In this review we discuss the roles of Hsp 27, 60, 70, and 90 in aging and male infertility and have concluded that these particular HSPs can be used as a molecular markers for mitochondrially- mediated apoptosis, aging and male infertility.
Keyword
MeSH Terms
Figure
Reference
-
1. De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999; 11:1–12.
Article2. Li Z, Srivastava P. Heat-shock proteins. Curr Protoc Immunol. 2004; 02. Appendix 1:Appendix 1T.3. Simar D, Ruell P, Caillaud C. Hsp responses to exercising in a warm environment. [Internet]. Doha: ASPETAR;c2013. cited 2014 Jul 20. Available from: http://www.aspetar.com/ResearchEducationCentre/HSPResponses.aspx.4. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997; 91:479–489.
Article5. Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med. 2005; 9:51–58.
Article6. Chandra D, Choy G, Tang DG. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J Biol Chem. 2007; 282:31289–31301.7. Zirkin BR, Chen H. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000; 63:977–981.8. Kimura M, Itoh N, Takagi S, Sasao T, Takahashi A, Masumori N, et al. Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J Androl. 2003; 24:185–191.
Article9. Meinhardt A, Wilhelm B, Seitz J. Expression of mitochondrial marker proteins during spermatogenesis. Hum Reprod Update. 1999; 5:108–119.10. Adly MA, Assaf HA, Hussein MR. Heat shock protein 27 expression in the human testis showing normal and abnormal spermatogenesis. Cell Biol Int. 2008; 32:1247–1255.
Article11. Werner A, Meinhardt A, Seitz J, Bergmann M. Distribution of heat-shock protein 60 immunoreactivity in testes of infertile men. Cell Tissue Res. 1997; 288:539–544.
Article12. Boulanger J, Faulds D, Eddy EM, Lingwood CA. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility. J Cell Physiol. 1995; 165:7–17.
Article13. Miller D, Brough S, al-Harbi O. Characterization and cellular distribution of human spermatozoal heat shock proteins. Hum Reprod. 1992; 7:637–645.
Article14. Kamaruddin M, Kroetsch T, Basrur PK, Hansen PJ, King WA. Immunolocalization of heat shock protein 70 in bovine spermatozoa. Andrologia. 2004; 36:327–334.
Article15. Huang SY, Tam MF, Hsu YT, Lin JH, Chen HH, Chuang CK, et al. Developmental changes of heat-shock proteins in porcine testis by a proteomic analysis. Theriogenology. 2005; 64:1940–1955.
Article16. Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, et al. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci U S A. 1996; 93:3264–3268.
Article17. Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I. Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J Biol Chem. 1991; 266:10099–10103.
Article18. Lee SJ. Expression of HSP86 in male germ cells. Mol Cell Biol. 1990; 10:3239–3242.
Article19. Erata GO, Koçak Toker N, Durlanik O, Kadioğlu A, Aktan G, Aykaç Toker G. The role of heat shock protein 70 (Hsp 70) in male infertility: is it a line of defense against sperm DNA fragmentation? Fertil Steril. 2008; 90:322–327.
Article20. Feng HL, Sandlow JI, Sparks AE. Decreased expression of the heat shock protein hsp70-2 is associated with the pathogenesis of male infertility. Fertil Steril. 2001; 76:1136–1139.
Article21. Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985; 82:6455–6459.
Article22. Sarge KD, Cullen KE. Regulation of hsp expression during rodent spermatogenesis. Cell Mol Life Sci. 1997; 53:191–197.
Article23. Rong C, Han J, Du Z. Expression of heat shock protein 90β and its regulation in the reproductive system of male mice. Nan Fang Yi Ke Da Xue Xue Bao. 2013; 33:491–495.24. Moore SK, Kozak C, Robinson EA, Ullrich SJ, Appella E. Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem. 1989; 264:5343–5351.
Article25. Ohsako S, Bunick D, Hayashi Y. Immunocytochemical observation of the 90 KD heat shock protein (HSP90): high expression in primordial and pre-meiotic germ cells of male and female rat gonads. J Histochem Cytochem. 1995; 43:67–76.26. Ecroyd H, Jones RC, Aitken RJ. Tyrosine phosphorylation of HSP-90 during mammalian sperm capacitation. Biol Reprod. 2003; 69:1801–1807.27. Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995; 11:441–469.
Article28. Liu Z, Wang G, Pan Y, Zhu C. Expression of androgen receptor and heat shock protein 90alpha in the testicular biopsy specimens of infertile patients with spermatogenic arrest. Zhonghua Nan Ke Xue. 2004; 10:662–666.29. Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007; 43:477–503.
Article30. Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005; 126:365–379.
Article31. Macario AJ, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005; 06. 353:1489–1501.
Article32. Soti C, Csermely P. Chaperones come of age. Cell Stress Chaperones. 2002; 7:186–190.33. Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997; 390:30.
Article34. Tower J. Hsps and aging. Trends Endocrinol Metab. 2009; 20:216–222.35. Jonak C, Klosner G, Trautinger F. Heat shock proteins in the skin. Int J Cosmet Sci. 2006; 28:233–241.
Article36. Préville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, et al. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res. 1999; 247:61–78.
Article37. Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002; 21:5164–5172.
Article38. Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998; 18:667–682.
Article39. Merendino AM, Paul C, Vignola AM, Costa MA, Melis M, Chiappara G, et al. Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: possible implication in asthma. Cell Stress Chaperones. 2002; 7:269–280.
Article40. Bryantsev AL, Kurchashova SY, Golyshev SA, Polyakov VY, Wunderink HF, Kanon B, et al. Regulation of stress-induced intracellular sorting and chaperone function of Hsp27 (HspB1) in mammalian cells. Biochem J. 2007; 407:407–417.
Article41. Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson's disease. Neuroreport. 1999; 10:2273–2276.42. Cappello F, Conway de Macario E, Marino Gammazza A, Bonaventura G, Carini F, Czarnecka AM, et al. Hsp60 and human aging: Les liaisons dangereuses. Front Biosci (Landmark Ed). 2013; 18:626–637.43. Spector NL, Mehlen P, Ryan C, Hardy L, Samson W, Levine H, et al. Regulation of the 28 kDa heat shock protein by retinoic acid during differentiation of human leukemic HL-60 cells. FEBS Lett. 1994; 337:184–188.44. Proctor CJ, Soti C, Boys RJ, Gillespie CS, Shanley DP, Wilkinson DJ, et al. Modelling the actions of chaperones and their role in ageing. Mech Ageing Dev. 2005; 126:119–131.
Article45. Buchner J. Hsp90 & Co. - a holding for folding. Trends Biochem Sci. 1999; 24:136–141.46. Rao DV, Watson K, Jones GL. Age-related attenuation in the expression of the major heat shock proteins in human peripheral lymphocytes. Mech Ageing Dev. 1999; 107:105–118.
Article47. Zhang HJ, Drake VJ, Morrison JP, Oberley LW, Kregel KC. Selected contribution: differential expression of stress-related genes with aging and hyperthermia. J Appl Physiol (1985). 2002; 92:1762–1769. discussion 1749.
Article48. Stolzing A, Sethe S, Scutt AM. Stressed stem cells: Temperature response in aged mesenchymal stem cells. Stem Cells Dev. 2006; 15:478–487.
Article49. Faassen AE, O'Leary JJ, Rodysill KJ, Bergh N, Hallgren HM. Diminished heat-shock protein synthesis following mitogen stimulation of lymphocytes from aged donors. Exp Cell Res. 1989; 183:326–334.
Article50. Hunter T, Poon RY. Cdc37: a protein kinase chaperone? Trends Cell Biol. 1997; 7:157–161.
Article51. Holt SE, Aisner DL, Baur J, Tesmer VM, Dy M, Ouellette M, et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999; 13:817–826.
Article52. Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002; 3:257–264.53. Ferlin A, Speltra E, Patassini C, Pati MA, Garolla A, Caretta N, et al. Heat shock protein and heat shock factor expression in sperm: relation to oligozoospermia and varicocele. J Urol. 2010; 183:1248–1252.
Article54. Mazzoli S, Cai T, Addonisio P, Bechi A, Mondaini N, Bartoletti R. Chlamydia trachomatis infection is related to poor semen quality in young prostatitis patients. Eur Urol. 2010; 57:708–714.
Article55. Venkataraman S, Wagner BA, Jiang X, Wang HP, Schafer FQ, Ritchie JM, et al. Overexpression of manganese superoxide dismutase promotes the survival of prostate cancer cells exposed to hyperthermia. Free Radic Res. 2004; 38:1119–1132.
Article56. Davidson JF, Schiestl RH. Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol Cell Biol. 2001; 21:8483–8489.57. Heise K, Puntarulo S, Pörtner HO, Abele D. Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternula elliptica (King and Broderip) under heat stress. Comp Biochem Physiol C Toxicol Pharmacol. 2003; 134:79–90.
Article58. Qian L, Song X, Ren H, Gong J, Cheng S. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 2004; 9:281–293.
Article59. Skulachev VP, Longo VD. Aging as a mitochondria-mediated atavistic program: can aging be switched off? Ann N Y Acad Sci. 2005; 1057:145–164.
Article60. Haak JL, Buettner GR, Spitz DR, Kregel KC. Aging augments mitochondrial susceptibility to heat stress. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R812–R820.
Article61. Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004; 101:7663–7668.
Article62. Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002; 12:712–723.
Article63. Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, et al. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006; 2:e115.
Article64. Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994; 91:10771–10778.
Article65. Ames BA, Shingenaga MK, Park EM. Davies KJA, editor. Oxidative damage and repair, chemical, biological and medical aspects. Elmstad, New York: Pergamon;1991. p. 181–187.66. Laganiere S, Yu BP. Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology. 1993; 39:7–18.
Article67. Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006; 11:116–128.
Article68. Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones. 2002; 7:309–316.
Article69. Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006; 41:653–657.
Article70. Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001; 36:341–352.
Article71. Imao M, Nagaki M, Moriwaki H. Dual effects of heat stress on tumor necrosis factor-alpha-induced hepatocyte apoptosis in mice. Lab Invest. 2006; 86:959–967.72. Sachidhanandam SB, Lu J, Low KS, Moochhala SM. Herbimycin A attenuates apoptosis during heat stress in rats. Eur J Pharmacol. 2003; 474:121–128.
Article73. Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003; 8:61–70.74. Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996; 271:16510–16514.75. Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, et al. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001; 6:49–58.
Article76. Moriyama-Gonda N, Igawa M, Shiina H, Urakami S, Wada Y, Terashima M. Heat-induced cellular damage and tolerance in combination with adriamycin for the PC-3 prostate cancer cell line: relationships with cytotoxicity, reactive oxygen species and heat shock protein 70 expression. Eur Urol. 2000; 38:235–240.77. Pallepati P, Averill-Bates DA. Mild thermotolerance induced at 40℃ protects HeLa cells against activation of death receptor-mediated apoptosis by hydrogen peroxide. Free Radic Biol Med. 2011; 50:667–679.78. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–257.
Article79. Steller H. Mechanisms and genes of cellular suicide. Science. 1995; 267:1445–1449.
Article80. Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY). 2012; 4:330–349.
Article81. Wickman G, Julian L, Olson MF. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012; 19:735–742.
Article82. Ai X, Butts B, Vora K, Li W, Tache-Talmadge C, Fridman A, et al. Generation and characterization of antibodies specific for caspase-cleaved neo-epitopes: a novel approach. Cell Death Dis. 2011; 2:e205.
Article83. Lüthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 2007; 14:641–650.
Article84. Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, et al. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999; 18:2049–2056.
Article85. Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008; 22:1427–1438.
Article86. Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006; 5:2592–2601.
Article87. Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000; 20:7146–7159.
Article88. Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004; 23:2907–2918.
Article89. Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther. 2007; 6:299–308.
Article90. Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007; 67:616–625.
Article91. Compton SA, Elmore LW, Haydu K, Jackson-Cook CK, Holt SE. Induction of nitric oxide synthase-dependent telomere shortening after functional inhibition of Hsp90 in human tumor cells. Mol Cell Biol. 2006; 26:1452–1462.
Article92. Gurbuxani S, Bruey JM, Fromentin A, Larmonier N, Parcellier A, Jäättelä M, et al. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene. 2001; 20:7478–7485.
Article93. Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, et al. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005; 25:899–910.
Article94. Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell. 2006; 17:886–894.
Article95. Liao W, Li X, Mancini M, Chan L. Proteasome inhibition induces differential heat shock protein response but not unfolded protein response in HepG2 cells. J Cell Biochem. 2006; 99:1085–1095.
Article96. Gusarova V, Caplan AJ, Brodsky JL, Fisher EA. Apoprotein B degradation is promoted by the molecular chaperones hsp90 and hsp70. J Biol Chem. 2001; 276:24891–24900.
Article97. Garrido C, Solary E. A role of HSPs in apoptosis through "protein triage"? Cell Death Differ. 2003; 10:619–620.
Article98. Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004; 279:51490–51499.
Article99. Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000; 19:4310–4322.
Article100. Kim HE, Jiang X, Du F, Wang X. PHAPI, CAS, and Hsp70 promote apoptosome formation by preventing Apaf-1 aggregation and enhancing nucleotide exchange on Apaf-1. Mol Cell. 2008; 30:239–247.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Hyperthermia-induced Apoptosis is Independent upon DNA Strand Breaks in Human Lymphoid Cells
- The Role of Heat Shock Protein in Perinatal Fields