Tuberc Respir Dis.
2010 Dec;69(6):456-464.
The Effect of Gefitinib on Immune Response of Human Peripheral Blood Monocyte-Derived Dendritic Cells
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea. leemk@pusan.ac.kr
Abstract
- BACKGROUND
Synergistic antitumor effects of the combined chemoimmunotherapy based on dendritic cells have been reported recently. The aim of this study is to search new applicability of gefitinib into the combination treatment through the confirmation of gefitinib effects on the monocyte derived dendritic cells (moDCs); most potent antigen presenting cell (APC).
METHODS
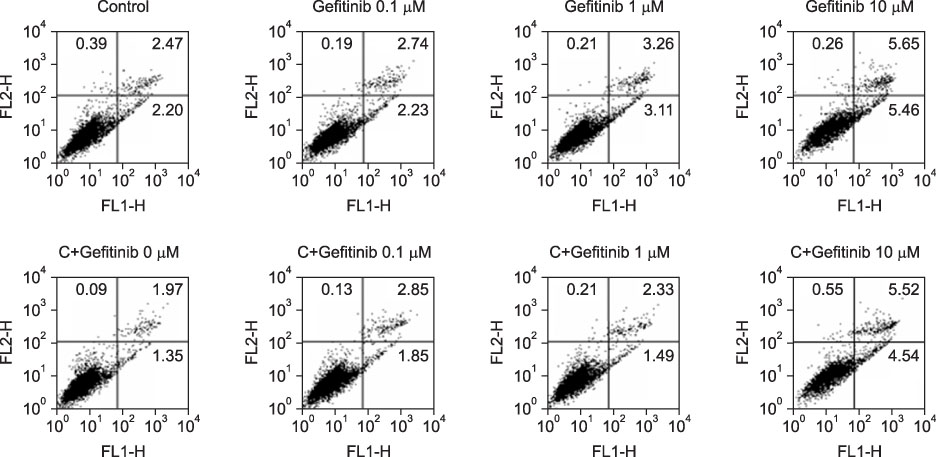
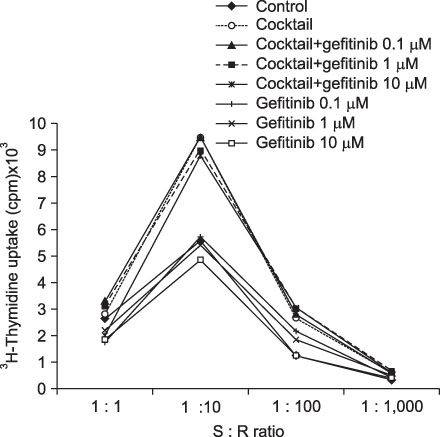
Immature and mature monocyte-derived dendritic cell (im, mMoDC)s were generated from peripheral blood monocyte (PBMC) in Opti-MEM culture medium supplemented with IL-4, GM-CSF and cocktail, consisting of TNF-alpha (10 ng/mL), IL-1beta (10 ng/mL), IL-6 (1,000 U/mL) and PGE2 (1 micro/mL). Various concentrations of gefitinib also added on day 6 to see the influence on immature and mature MoDCs. Immunophenotyping of DCs under the gefitinib was performed by using monoclonal antibodies (CD14, CD80, CD83, CD86, HLA-ABC, HLA-DR). Supernatant IL-12 production and apoptosis of DCs was evaluated. And MLR assay with [3H]-thymidine uptake assay was done.
RESULTS
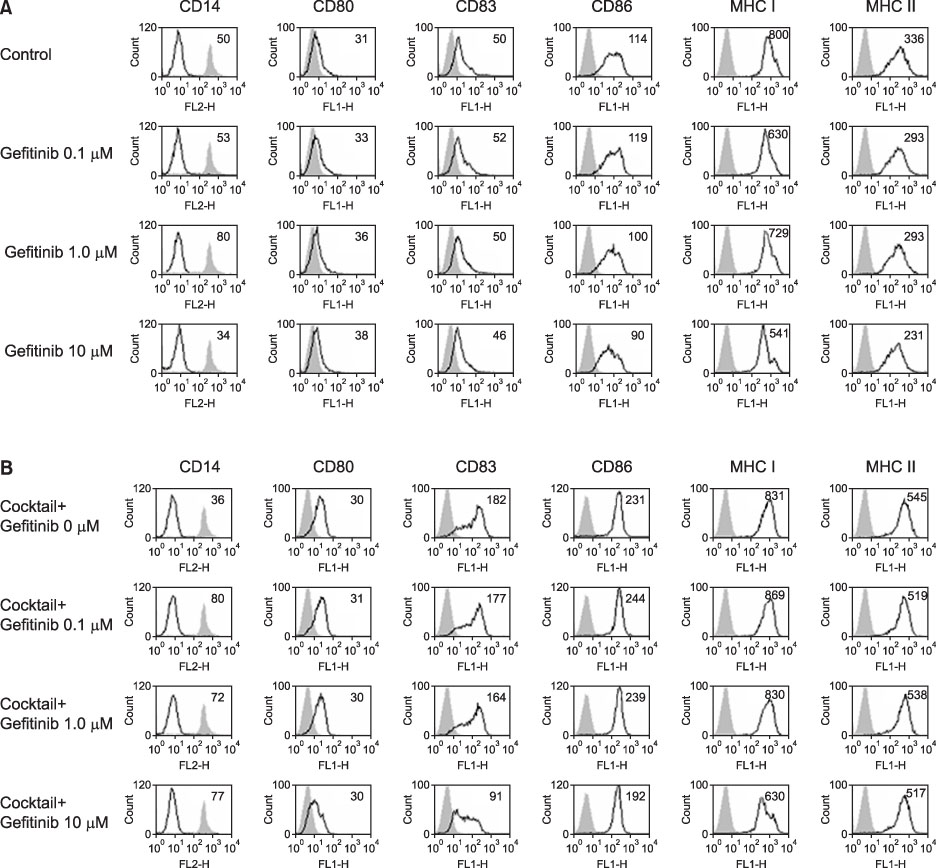
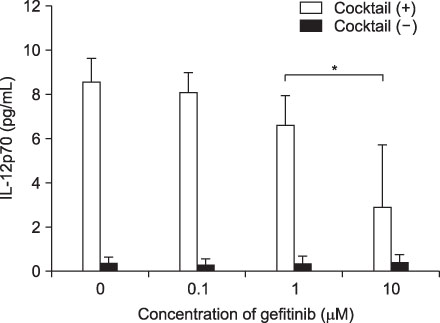
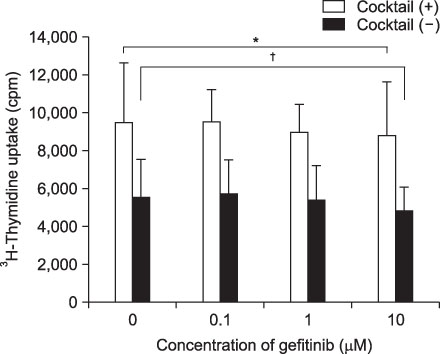
Expression of CD83, MHC I were decreased in mMoDCs and MHC I was decreased in imMoDCs under gefitinib. IL-12 production from mMoDCs was decreased under 10 microM of gefitinib sinificantly. Differences of T cell proliferation capacity were not observed in each concentration of geftinib.
CONCLUSION
In spite of decreased expressions of some dendritic cell surface molecules and IL-12 production under 10 microM of gefitinib, significant negative influences of gefitinib in antigen presenting capacity and T cell stimulation were not observed.
Keyword
MeSH Terms
-
Antibodies, Monoclonal
Apoptosis
Cell Proliferation
Dendritic Cells
Dinoprostone
Granulocyte-Macrophage Colony-Stimulating Factor
Humans
Immunophenotyping
Interleukin-12
Interleukin-4
Interleukin-6
Monocytes
Quinazolines
Tumor Necrosis Factor-alpha
Antibodies, Monoclonal
Dinoprostone
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-12
Interleukin-4
Interleukin-6
Quinazolines
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.2. Emens LA, Machiels JP, Reilly RT, Jaffee EM. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001. 3:77–84.3. Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer. 2005. 5:397–405.4. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008. 8:59–73.5. Ménard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008. 57:1579–1587.6. Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008. 20:545–557.7. Slingluff CL Jr, Engelhard VH, Ferrone S. Peptide and dendritic cell vaccines. Clin Cancer Res. 2006. 12:2342s–2345s.8. Nemunaitis J, Sterman D, Jablons D, Smith JW 2nd, Fox B, Maples P, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004. 96:326–331.9. Nair SK, Hull S, Coleman D, Gilboa E, Lyerly HK, Morse MA. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer. 1999. 82:121–124.10. Itoh T, Ueda Y, Kawashima I, Nukaya I, Fujiwara H, Fuji N, et al. Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother. 2002. 51:99–106.11. Ueda Y, Itoh T, Nukaya I, Kawashima I, Okugawa K, Yano Y, et al. Dendritic cell-based immunotherapy of cancer with carcinoembryonic antigen-derived, HLA-A24-restricted CTL epitope: Clinical outcomes of 18 patients with metastatic gastrointestinal or lung adenocarcinomas. Int J Oncol. 2004. 24:909–917.12. Kontani K, Taguchi O, Ozaki Y, Hanaoka J, Sawai S, Inoue S, et al. Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int J Mol Med. 2003. 12:493–502.13. Belardelli F, Ferrantini M, Parmiani G, Schlom J, Garaci E. International meeting on cancer vaccines: how can we enhance efficacy of therapeutic vaccines? Cancer Res. 2004. 64:6827–6830.14. O'Mahony D, Kummar S, Gutierrez ME. Non-small-cell lung cancer vaccine therapy: a concise review. J Clin Oncol. 2005. 23:9022–9028.15. Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006. 12:878–887.16. Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006. 58:975–990.17. Choi GS, Lee MH, Kim SK, Kim CS, Lee HS, Im MW, et al. Combined treatment of an intratumoral injection of dendritic cells and systemic chemotherapy (paclitaxel) for murine fibrosarcoma. Yonsei Med J. 2005. 46:835–842.18. Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004. 34:336–344.19. Nakashima H, Tasaki A, Kubo M, Kuroki H, Matsumoto K, Tanaka M, et al. Effects of docetaxel on antigen presentation-related functions of human monocyte-derived dendritic cells. Cancer Chemother Pharmacol. 2005. 55:479–487.20. Casati A, Zimmermann VS, Benigni F, Bertilaccio MT, Bellone M, Mondino A. The immunogenicity of dendritic cell-based vaccines is not hampered by doxorubicin and melphalan administration. J Immunol. 2005. 174:3317–3325.21. Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003. 63:4490–4496.22. Correale P, Cusi MG, Del Vecchio MT, Aquino A, Prete SP, Tsang KY, et al. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol. 2005. 175:820–828.23. Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, et al. United states food and drug administration drug approval summary: gefitinib (ZD 1839; Iressa) tablets. Clin Cancer Res. 2004. 10:1212–1218.24. Rosell R, Viteri S, Molina MA, Benlloch S, Taron M. Epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in advanced nonsmall-cell lung cancer. Curr Opin Oncol. 2010. 22:112–120.25. Chang A, Parikh P, Thongprasert S, Tan EH, Perng RP, Ganzon D, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL study. J Thorac Oncol. 2006. 1:847–855.26. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008. 372:1809–1818.27. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.28. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.29. Palucka AK, Ueno H, Fay J, Banchereau J. Dendritic cells: a critical player in cancer therapy? J Immunother. 2008. 31:793–805.30. Fujii S, Takayama T, Asakura M, Aki K, Fujimoto K, Shimizu K. Dendritic cell-based cancer immunotherapies. Arch Immunol Ther Exp (Warsz). 2009. 57:189–198.31. Choi YJ, Rho JK, Jeon BS, Choi SJ, Park SC, Lee SS, et al. Combined inhibition of IGFR enhances the effects of gefitinib in H1650: a lung cancer cell line with EGFR mutation and primary resistance to EGFR-TK inhibitors. Cancer Chemother Pharmacol. 2010. 66:381–388.32. Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008. 7:9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Expression of Intracellular HLA-DM but Not on the Surface of Blood Monocyte-derived Dendritic Cells During Maturation
- Analysis of methods for the generation of dendritic cells from human peripheral blood monocytes
- Effect of Human Cytomegalovirus on Human Monocyte-derived Dendritic Cells
- IL-12 Production in Lipopolysaccharide- and Dermatophagoides pteronyssinus- Copulsed Monocyte-derived Dendritic Cells
- Culture of Dendritic Cell from Normal Peripheral Blood Monocyte and Its Anti-tumor Immune Activity When Pulsed by Renal Cell Carcinoma Cell Line: In vitro Study