Lab Anim Res.
2015 Sep;31(3):134-138. 10.5625/lar.2015.31.3.134.
Curcumin treatment recovery the decrease of protein phosphatase 2A subunit B induced by focal cerebral ischemia in Sprague-Dawley rats
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- KMID: 2312130
- DOI: http://doi.org/10.5625/lar.2015.31.3.134
Abstract
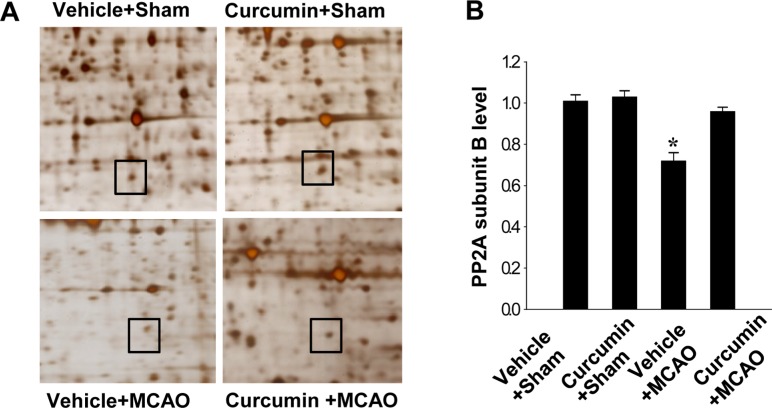
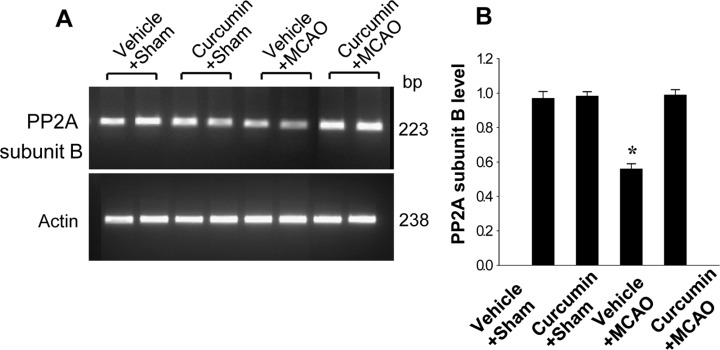
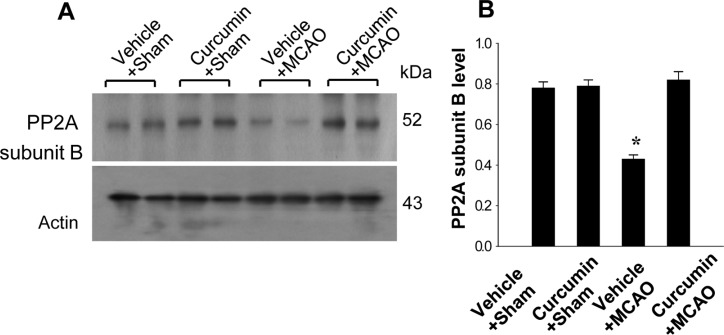
- Curcumin provides various biological effects through its anti-inflammatory and antioxidant properties. Moreover, curcumin exerts a neuroprotective effect against ischemic condition-induced brain damage. Protein phosphatase 2A (PP2A) is a ubiquitous serine and threonine phosphatase with various cell functions and broad substrate specificity. Especially PP2A subunit B plays an important role in nervous system. This study investigated whether curcumin regulates PP2A subunit B expression in focal cerebral ischemia. Cerebral ischemia was induced surgically by middle cerebral artery occlusion (MCAO). Adult male rats were injected with either vehicle or curcumin (50 mg/kg) 1 h after MCAO and cerebral cortex tissues were isolated 24 h after MCAO. A proteomics study, reverse transverse-PCR and Western blot analyses were performed to examine PP2A subunit B expression levels. We identified a reduction in PP2A subunit B expression in MCAO-operated animals using a proteomic approach. However, curcumin treatment prevented injury-induced reductions in PP2A subunit B levels. Reverse transverse-PCR and Western blot analyses confirmed that curcumin treatment attenuated the injury-induced reduction in PP2A subunit B levels. These findings can suggest that the possibility that curcumin maintains levels of PP2A subunit B in response to cerebral ischemia, which likely contributes to the neuroprotective function of curcumin in cerebral ischemic injury.
MeSH Terms
-
Adult
Animals
Blotting, Western
Brain
Brain Ischemia*
Cerebral Cortex
Curcumin*
Humans
Infarction, Middle Cerebral Artery
Male
Nervous System
Neuroprotective Agents
Phosphoprotein Phosphatases
Protein Phosphatase 2*
Proteomics
Rats
Rats, Sprague-Dawley*
Serine
Substrate Specificity
Curcumin
Neuroprotective Agents
Phosphoprotein Phosphatases
Protein Phosphatase 2
Serine
Figure
Reference
-
1. Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012; 60(2):208–212. PMID: 22122807.
Article2. Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr Med Chem. 2015; 22(10):1258–1277. PMID: 25666795.
Article3. Dutta S, Padhye S, Priyadarsini KI, Newton C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett. 2005; 15(11):2738–2744. PMID: 15878268.
Article4. Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit. 2007; 13(12):BR286–BR292. PMID: 18049430.5. Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008; 1204:16–23. PMID: 18329634.
Article6. Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, Ha I, Han JS. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005; 82(6):831–838. PMID: 16273545.
Article7. Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005; 7(1-2):32–41. PMID: 15650394.8. Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006; 6(3):259–270. PMID: 16712454.
Article9. Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006; 25(2):278–287. PMID: 16170359.
Article10. Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002; 46(3):273–279. PMID: 12220971.
Article11. Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004; 74(8):969–985. PMID: 14672754.
Article12. Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005; 82(1):138–148. PMID: 16075466.
Article13. Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008; 1229:224–232. PMID: 18640105.
Article14. Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010; 35(3):374–379. PMID: 19774461.
Article15. Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001; 353(Pt 3):417–439. PMID: 11171037.
Article16. Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001; 13(1):7–16. PMID: 11257442.
Article17. Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000; 60(8):1225–1235. PMID: 11007961.
Article18. Stone SR, Hofsteenge J, Hemmings BA. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987; 26(23):7215–7220. PMID: 2827745.
Article19. Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999; 24(5):186–191. PMID: 10322434.
Article20. Sim AT. The regulation and function of protein phosphatases in the brain. Mol Neurobiol. 1991; 5(2-4):229–246. PMID: 1668387.
Article21. Strack S, Zaucha JA, Ebner FF, Colbran RJ, Wadzinski BE. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998; 392(4):515–527. PMID: 9514514.
Article22. Koh PO. Focal Cerebral Ischemia Reduces Protein Phosphatase 2A Subunit B Expression in Brain Tissue and HT22 Cells. Lab Anim Res. 2011; 27(1):73–76. PMID: 21826165.
Article23. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article24. Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000; 275(8):5535–5544. PMID: 10681533.25. Liu R, Wang JZ. Protein phosphatase 2A in Alzheimer's disease. Pathophysiology. 2009; 16(4):273–277. PMID: 19278841.
Article26. Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996; 17(6):1201–1207. PMID: 8982166.
Article27. Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, White CL 3rd. Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004; 63(10):1080–1091. PMID: 15535135.
Article28. Cancino GI, Toledo EM, Leal NR, Hernandez DE, Yévenes LF, Inestrosa NC, Alvarez AR. STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer's beta-amyloid deposits. Brain. 2008; 131:2425–2442. PMID: 18559370.29. Kanemaru K. [Immunotherapy targeting misfolded proteins in neurodegenerative disease]. Brain Nerve. 2013; 65(4):469–474. PMID: 23568995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Focal Cerebral Ischemia Reduces Protein Phosphatase 2A Subunit B Expression in Brain Tissue and HT22 Cells
- Decrease of protein phosphatase 2A subunit B by glutamate exposure in the cerebral cortex of neonatal rats
- Curcumin attenuates the middle cerebral artery occlusion-induced reduction in gamma-enolase expression in an animal model
- Epigallocatechin gallate restores the reduction of protein phosphatase 2 A subunit B caused by middle cerebral artery occlusion
- Changes in Evoked Potentials in Focal Cerebral Cortical Ischemia in the Rat