Korean J Physiol Pharmacol.
2014 Jun;18(3):225-231. 10.4196/kjpp.2014.18.3.225.
The Protective Role of TLR3 and TLR9 Ligands in Human Pharyngeal Epithelial Cells Infected with Influenza A Virus
- Affiliations
-
- 1Dalian Center for Disease Control and Prevention, Dalian 116021, China. 18940956816@163.com
- KMID: 2285514
- DOI: http://doi.org/10.4196/kjpp.2014.18.3.225
Abstract
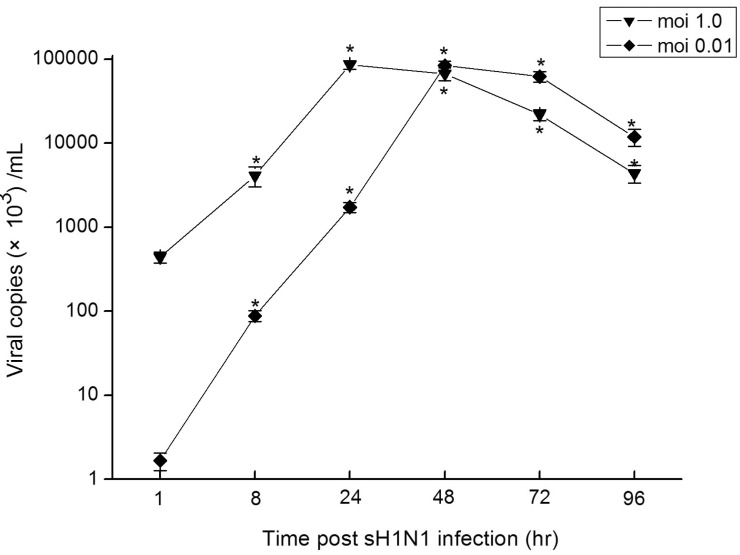
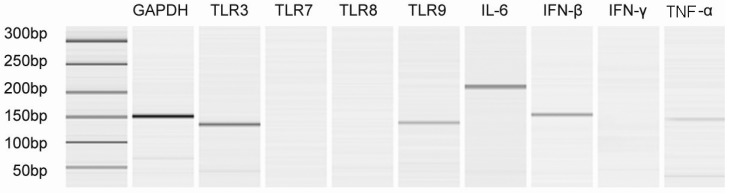
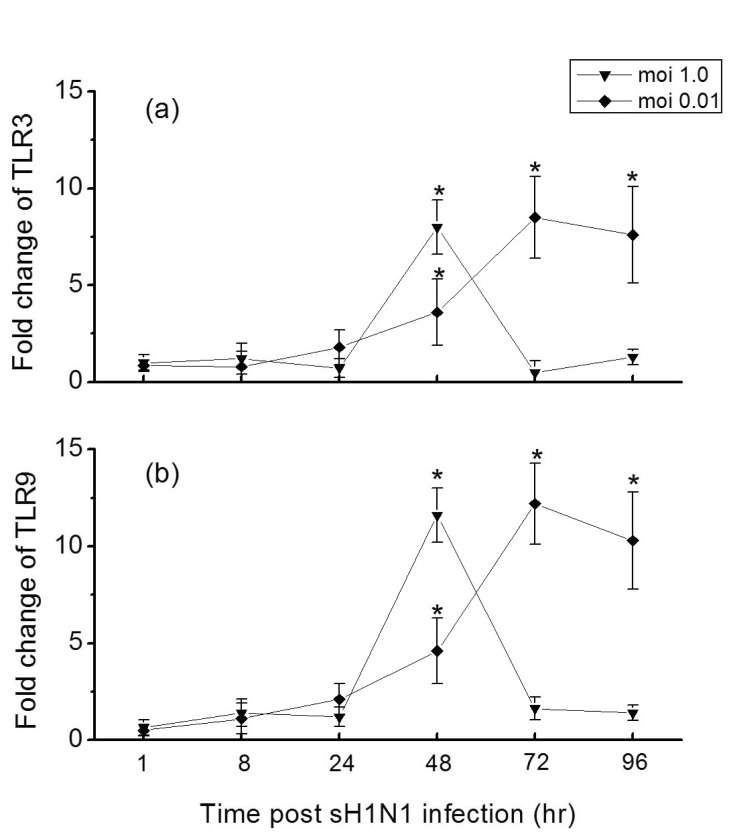
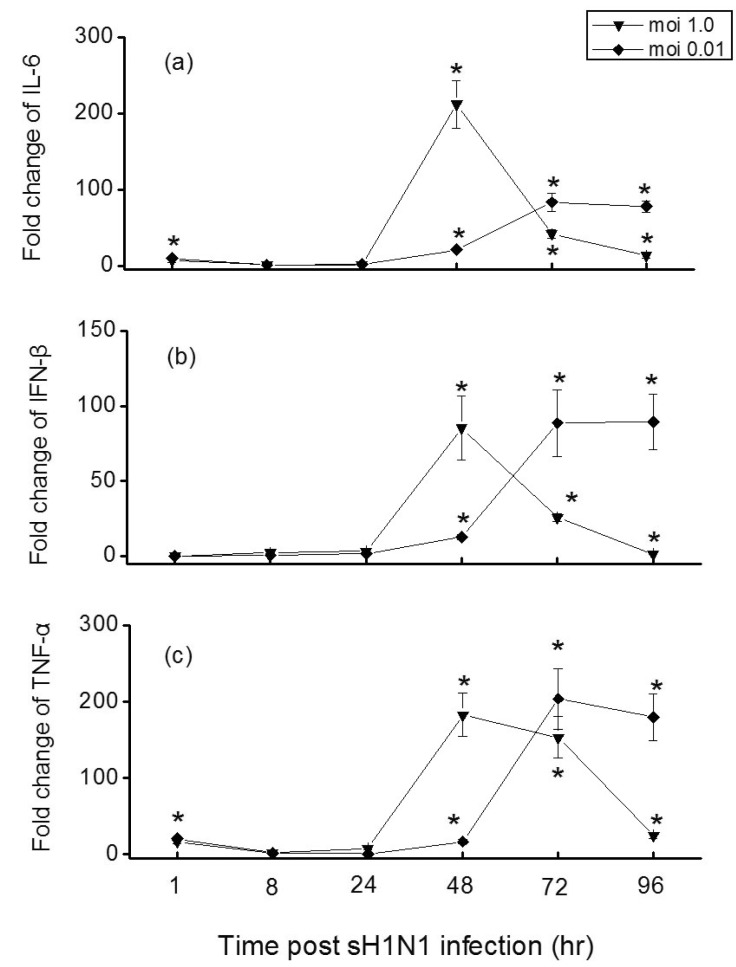
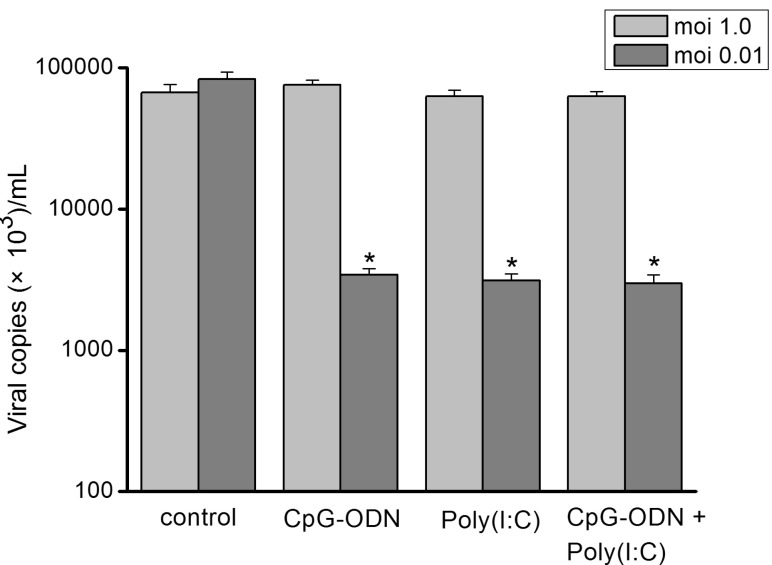
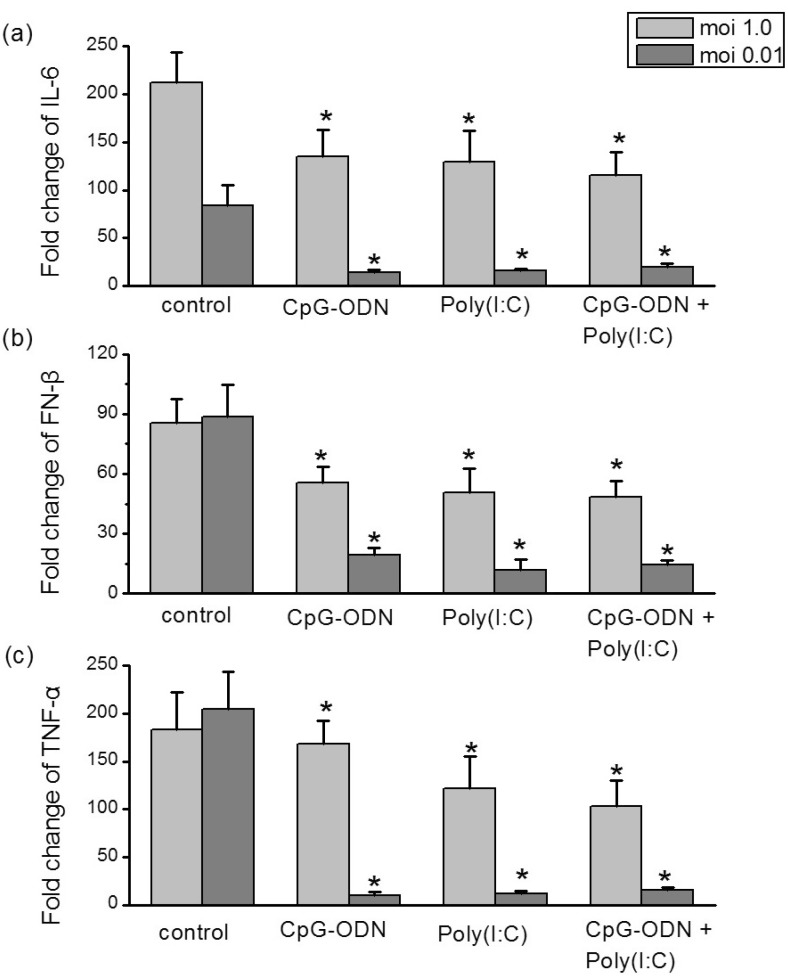
- In this study we aim to extensively investigate the anti-influenza virus immune responses in human pharyngeal epithelial cell line (Hep-2) and evaluate the protective role of Toll-like receptor (TLR) ligands in seasonal influenza A H1N1 (sH1N1) infections in vitro. We first investigated the expression of the TLRs and cytokines genes in resting and sH1N1 infected Hep-2 cells. Clear expressions of TLR3, TLR9, interleukin (IL)-6, tumour necrosis factor (TNF)-alpha and interferon (IFN)-beta were detected in resting Hep-2 cells. After sH1N1 infection, a ten-fold of TLR3 and TLR9 were elicited. Concomitant with the TLRs activation, transcriptional expression of IL-6, TNF-alpha and IFN-beta were significantly induced in sH1N1-infected cells. Pre-treatment of cells with poly I:C (an analog of viral double-stranded RNA) and CpG-ODN (a CpG-motif containing oligodeoxydinucleotide) resulted in a strong reduction of viral and cytokines mRNA expression. The results presented indicated the innate immune response activation in Hep-2 cells and affirm the antiviral role of Poly I:C and CpG-ODN in the protection against seasonal influenza A viruses.
MeSH Terms
-
Cytokines
Epithelial Cells*
Humans
Immunity, Innate
Influenza A virus*
Influenza, Human
Interferons
Interleukin-6
Interleukins
Ligands*
Necrosis
RNA, Messenger
Seasons
Toll-Like Receptors
Transcriptional Activation
Tumor Necrosis Factor-alpha
Cytokines
Interferons
Interleukin-6
Interleukins
Ligands
RNA, Messenger
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Miller M, Viboud C, Simonsen L, Olson DR, Russell C. Mortality and morbidity burden associated with A/H1N1pdm influenza virus: Who is likely to be infected, experience clinical symptoms, or die from the H1N1pdm 2009 pandemic virus? Version 2. PLoS Curr. 2009; 1:RRN1013. PMID: 20029607.2. Treanor JD. Influenza--the goal of control. N Engl J Med. 2007; 357:1439–1441. PMID: 17914046.3. Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis. 2006; 194(Suppl 2):S133–S138. PMID: 17163386.
Article4. Nayak DP, Hui EK, Barman S. Assembly and budding of influenza virus. Virus Res. 2004; 106:147–165. PMID: 15567494.
Article5. Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013-2014. MMWR Recomm Rep. 2013; 62:1–43.6. Couch RB. Prevention and treatment of influenza. N Engl J Med. 2000; 343:1778–1787. PMID: 11114318.
Article7. Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010; 7:440–451. PMID: 20542248.8. Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melén K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001; 12:171–180. PMID: 11325600.
Article9. Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006; 7:131–137. PMID: 16424890.
Article10. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384. PMID: 20404851.
Article11. Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004; 4:249–258. PMID: 15057783.
Article12. Wong JP, Christopher ME, Viswanathan S, Karpoff N, Dai X, Das D, Sun LQ, Wang M, Salazar AM. Activation of toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine. 2009; 27:3481–3483. PMID: 19200852.
Article13. Wang Y, Shan C, Ming S, Liu Y, Du Y, Jiang G. Immunoadjuvant effects of bacterial genomic DNA and CpG oligodeoxynucleotides on avian influenza virus subtype H5N1 inactivated oil emulsion vaccine in chicken. Res Vet Sci. 2009; 86:399–405. PMID: 18977008.
Article14. Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007; 178:3368–3372. PMID: 17339430.
Article15. Li J, Jeong MY, Bae JH, Shin YH, Jin M, Hang SM, Lee JC, Lee SJ, Park K. Toll-like receptor3-mediated induction of chemokines in salivary epithelial cells. Korean J Physiol Pharmacol. 2010; 14:235–240. PMID: 20827338.
Article16. Li H, Zhang J, Kumar A, Zheng M, Atherton SS, Yu FS. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology. 2006; 117:167–176. PMID: 16423052.
Article17. Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001; 14:778–809. PMID: 11585785.
Article18. Koerner I, Kochs G, Kalinke U, Weiss S, Staeheli P. Protective role of beta interferon in host defense against influenza A virus. J Virol. 2007; 81:2025–2030. PMID: 17151098.
Article19. Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001; 64:262–268. PMID: 11424113.
Article20. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005; 5:718–725. PMID: 16253889.
Article21. Rimmelzwaan GF, Baars M, van Beek R, van Amerongen G, Lövgren-Bengtsson K, Claas EC, Osterhaus AD. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997; 78:757–765. PMID: 9129647.
Article22. Xie H, Raybourne RB, Babu US, Lillehoj HS, Heckert RA. CpG-induced immunomodulation and intracellular bacterial killing in a chicken macrophage cell line. Dev Comp Immunol. 2003; 27:823–834. PMID: 12818639.
Article23. de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006; 12:1203–1207. PMID: 16964257.
Article24. Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, García-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006; 443:578–581. PMID: 17006449.
Article25. Li IW, Chan KH, To KW, Wong SS, Ho PL, Lau SK, Woo PC, Tsoi HW, Chan JF, Cheng VC, Zheng BJ, Chen H, Yuen KY. Differential susceptibility of different cell lines to swine-origin influenza A H1N1, seasonal human influenza A H1N1, and avian influenza A H5N1 viruses. J Clin Virol. 2009; 46:325–330. PMID: 19801200.
Article26. Kido H, Okumura Y, Takahashi E, Pan HY, Wang S, Chida J, Le TQ, Yano M. Host envelope glycoprotein processing proteases are indispensable for entry into human cells by seasonal and highly pathogenic avian influenza viruses. J Mol Genet Med. 2008; 3:167–175. PMID: 19565019.
Article27. Sen GC, Sarkar SN. Transcriptional signaling by double stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 2005; 16:1–24. PMID: 15733829.28. Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007; 13:552–559. PMID: 17479101.
Article29. Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002; 76:1071–1076. PMID: 11773383.
Article30. Zu M, Yang F, Zhou W, Liu A, Du G, Zheng L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012; 94:217–224. PMID: 22521753.
Article31. Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001; 64:262–268. PMID: 11424113.
Article32. Svitek N, Rudd PA, Obojes K, Pillet S, von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008; 376:53–59. PMID: 18420248.
Article33. Brydon EW, Morris SJ, Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev. 2005; 29:837–850. PMID: 16102605.
Article34. Bauer RN, Brighton LE, Mueller L, Xiang Z, Rager JE, Fry RC, Peden DB, Jaspers I. Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J Allergy Clin Immunol. 2012; 130:958–967. PMID: 23021143.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Toll-like receptors 3, 7, 9 and cytokines in feline infectious peritonitis virus-infected CRFK cells and feline peripheral monocytes
- Variable localization of Toll-like receptors in human fallopian tube epithelial cells
- Clinical Use of Tamiflu (Oseltamivir)
- Effects of Hantaan Virus and IFN-gammaon Induction of Surface ICAM-1 in Primary Cultured Buman Nasal Epithelial Cells and Human Lung Fibroblasts
- Prevention and Treatment of Influenza