Korean J Physiol Pharmacol.
2012 Dec;16(6):447-453. 10.4196/kjpp.2012.16.6.447.
Multiple Signaling Molecules are Involved in Expression of CCL2 and IL-1beta in Response to FSL-1, a Toll-Like Receptor 6 Agonist, in Macrophages
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Pusan National University, Yangsan 626-870, Korea. koanhoi@pusan.ac.kr
- 2Department of Neurosurgery, School of Medicine, Konkuk University, Chungju 380-704, Korea.
- 3Laboratory of Microbiology, College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 2285454
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.447
Abstract
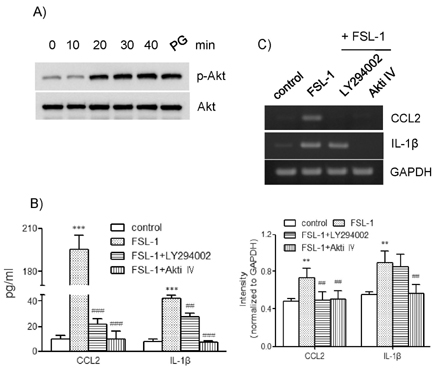
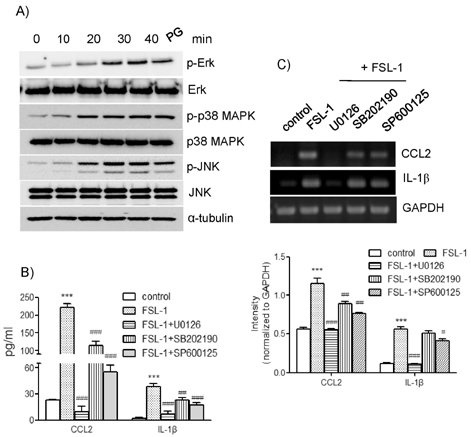
- TLR6 forms a heterodimer with TLR2 and TLR4. While proinflammatory roles of TLR2 and TLR4 are well documented, the role of TLR6 in inflammation is poorly understood. In order to understand mechanisms of action of TLR6 in inflammatory responses, we investigated the effects of FSL-1, the TLR6 ligand, on expression of chemokine CCL2 and cytokine IL-1beta and determined cellular factors involved in FSL-1-mediated expression of CCL2 and IL-1beta in mononuclear cells. Exposure of human monocytic leukemia THP-1 cells to FSL-1 resulted not only in enhanced secretion of CCL2 and IL-1beta, but also profound induction of their gene transcripts. Expression of CCL2 was abrogated by treatment with OxPAPC, a TLR-2/4 inhibitor, while treatment with OxPAPC resulted in partially inhibited expression of IL-1beta. Treatment with FSL-1 resulted in enhanced phosphorylation of Akt and mitogen-activated protein kinases and activation of protein kinase C. Treatment with pharmacological inhibitors, including SB202190, SP6001250, U0126, Akt inhibitor IV, LY294002, GF109203X, and RO318220 resulted in significantly attenuated FSL-1-mediated upregulation of CCL2 and IL-1beta. Our results indicate that activation of TLR6 will trigger inflammatory responses by upregulating expression of CCL2 and IL-1beta via TLR-2/4, protein kinase C, PI3K-Akt, and mitogen-activated protein kinases.
Keyword
MeSH Terms
-
Butadienes
Chemokine CCL2
Chromones
Humans
Imidazoles
Indoles
Inflammation
Leukemia
Macrophages
Maleimides
Mitogen-Activated Protein Kinases
Morpholines
Nitriles
Phosphatidylcholines
Phosphorylation
Protein Kinase C
Pyridines
Toll-Like Receptor 6
Toll-Like Receptors
Up-Regulation
Butadienes
Chemokine CCL2
Chromones
Imidazoles
Indoles
Maleimides
Mitogen-Activated Protein Kinases
Morpholines
Nitriles
Phosphatidylcholines
Protein Kinase C
Pyridines
Toll-Like Receptor 6
Toll-Like Receptors
Figure
Cited by 1 articles
-
FSL-1, a Toll-like Receptor 2/6 Agonist, Induces Expression of Interleukin-1α in the Presence of 27-hydroxycholesterol
Weon Heo, Sun-Mi Kim, Seong-Kug Eo, Byung-Yong Rhim, Koanhoi Kim
Korean J Physiol Pharmacol. 2014;18(6):475-480. doi: 10.4196/kjpp.2014.18.6.475.
Reference
-
1. Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999. 138:S534–S536.2. Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011. 108:Suppl 1. 4592–4598.3. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006. 124:783–801.4. Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004. 101:10679–10684.5. Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005. 115:3149–3156.6. Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Röschmann K, Jung G, Wiesmüller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2008. 83:692–701.7. Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010. 11:155–161.8. Nakao Y, Funami K, Kikkawa S, Taniguchi M, Nishiguchi M, Fukumori Y, Seya T, Matsumoto M. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J Immunol. 2005. 174:1566–1573.9. Into T, Fujita M, Okusawa T, Hasebe A, Morita M, Shibata K. Synergic effects of mycoplasmal lipopeptides and extracellular ATP on activation of macrophages. Infect Immun. 2002. 70:3586–3591.10. Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, Gullestad L, Damås JK. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008. 28:1909–1919.11. Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Med. 2004. 36:98–118.12. Young JL, Libby P, Schönbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002. 88:554–567.13. Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991. 88:1121–1127.14. Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999. 103:773–778.15. Schrader JW, Moyer C, Ziltener HJ, Reinisch CL. Release of the cytokines colony-stimulating factor-1, granulocyte-macrophage colony-stimulating factor, and IL-6 by cloned murine vascular smooth muscle cells. J Immunol. 1991. 146:3799–3808.16. Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci USA. 2002. 99:6280–6285.17. Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003. 23:656–660.18. Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004. 110:1678–1685.19. Kang SH, Lee JH, Choi KH, Rhim BY, Kim K. Roles of ERK and NF-kappaB in interleukin-8 expression in response to heat shock protein 22 in vascular smooth muscle cells. Korean J Physiol Pharmacol. 2008. 12:171–176.20. Seneviratne AN, Sivagurunathan B, Monaco C. Toll-like receptors and macrophage activation in atherosclerosis. Clin Chim Acta. 2012. 413:3–14.21. Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007. 30:1617–1623.22. Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012. 3:185.23. Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997. 275:665–668.24. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002. 2:489–501.25. El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 2003. 17:720–722.26. Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006. 13:816–825.27. Lee SA, Kim SM, Son YH, Lee CW, Chung SW, Eo SK, Rhim BY, Kim K. Peptidoglycan enhances secretion of monocyte chemoattractants via multiple signaling pathways. Biochem Biophys Res Commun. 2011. 408:132–138.28. Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J Cell Physiol. 2007. 210:667–675.29. Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal. 2005. 17:385–394.30. Cuschieri J, Billigren J, Maier RV. Endotoxin tolerance attenuates LPS-induced TLR4 mobilization to lipid rafts: a condition reversed by PKC activation. J Leukoc Biol. 2006. 80:1289–1297.31. Fronhofer V, Lennartz MR, Loegering DJ. Role of PKC isoforms in the Fc(gamma)R-mediated inhibition of LPS-stimulated IL-12 secretion by macrophages. J Leukoc Biol. 2006. 79:408–415.32. Sipma H, van der Zee L, van den Akker J, den Hertog A, Nelemans A. The effect of the PKC inhibitor GF109203X on the release of Ca2+ from internal stores and Ca2+ entry in DDT1 MF-2 cells. Br J Pharmacol. 1996. 119:730–736.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- FSL-1, a Toll-like Receptor 2/6 Agonist, Induces Expression of Interleukin-1alpha in the Presence of 27-hydroxycholesterol
- 7alpha-Hydroxycholesterol Elicits TLR6-Mediated Expression of IL-23 in Monocytic Cells
- Intracellular Signaling Pathways that Regulate Macrophage Chemokine Expression in Response to Mycobacterium abscessus
- Neonatal innate immunity and Toll-like receptor
- Interleukin-1beta Participates in the Development of Pneumococcal Acute Lung Injury and Death by Promoting Alveolar Microvascular Leakage