Korean J Physiol Pharmacol.
2014 Dec;18(6):475-480. 10.4196/kjpp.2014.18.6.475.
FSL-1, a Toll-like Receptor 2/6 Agonist, Induces Expression of Interleukin-1alpha in the Presence of 27-hydroxycholesterol
- Affiliations
-
- 1Department of Pharmacology, Pusan National University School of Medicine, Yangsan, 626-870, Korea. koanhoi@pusan.ac.kr
- 2College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Iksan, 570-752, Korea.
- KMID: 2285549
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.475
Abstract
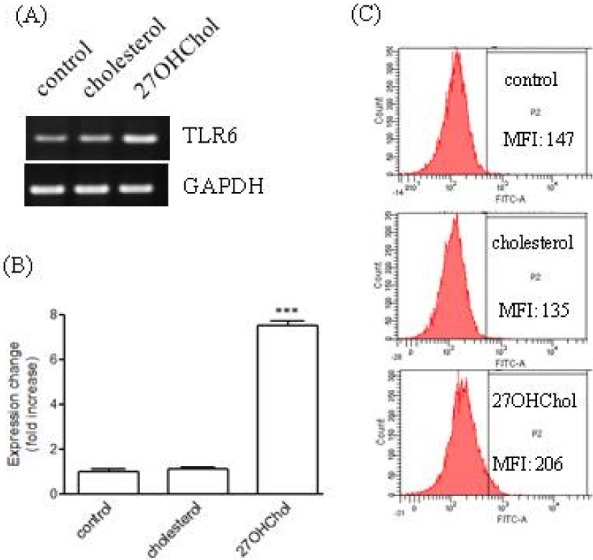
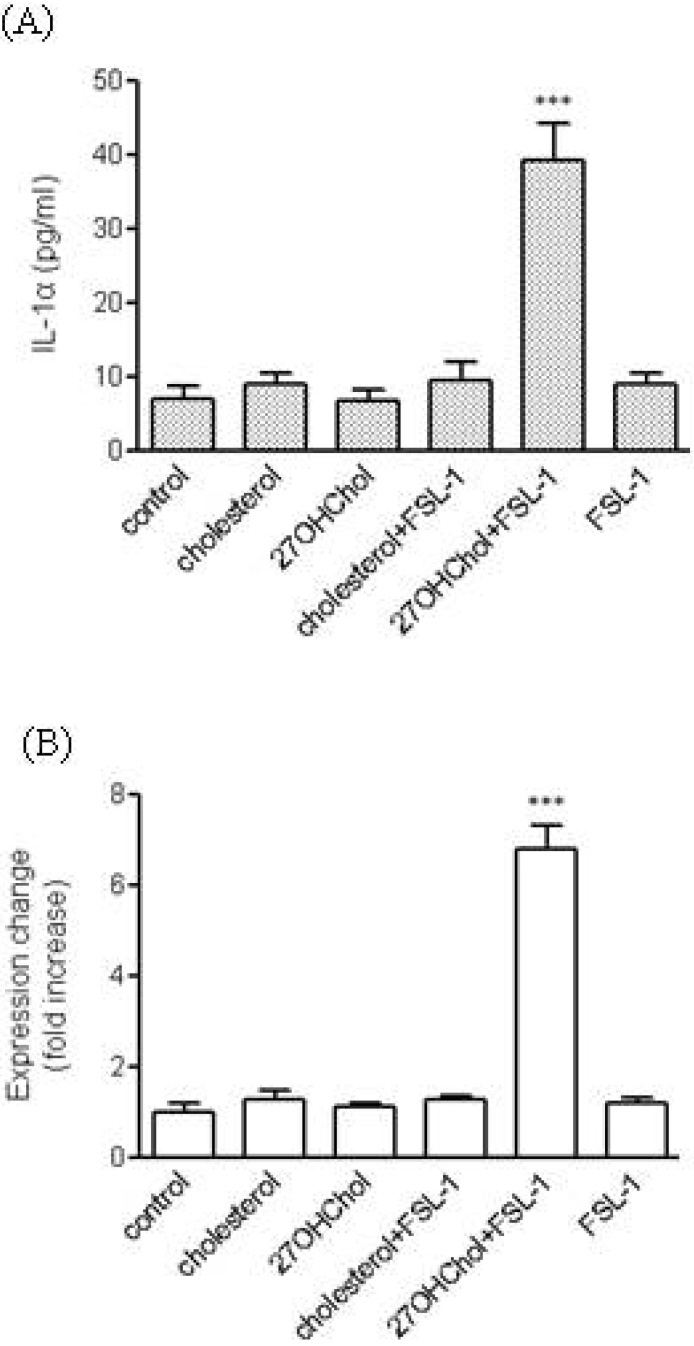
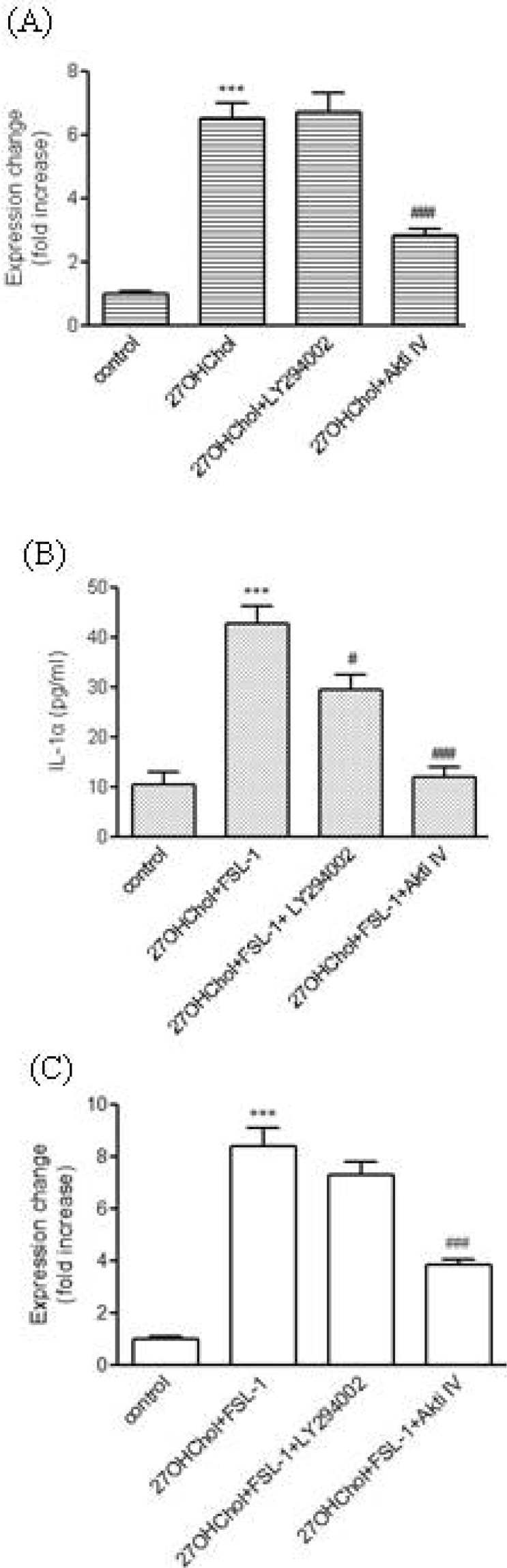
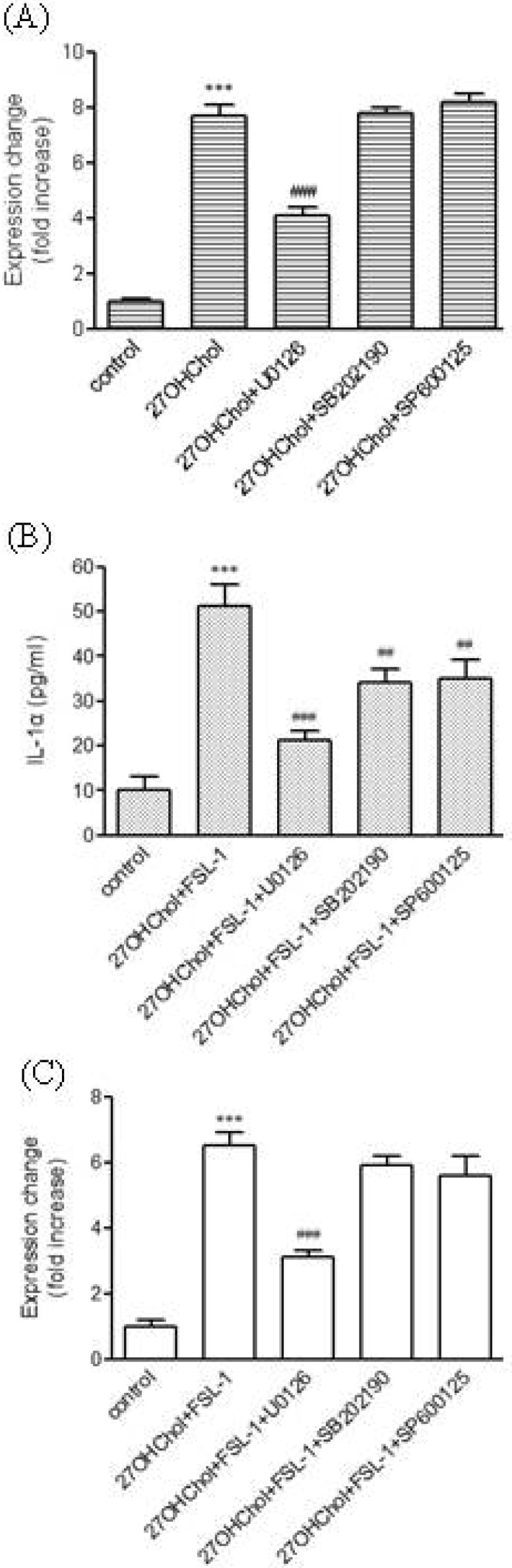
- We investigated the question of whether cholesterol catabolite can influence expression of inflammatory cytokines via Toll-like receptors (TLR) in monocytic cells. Treatment of THP-1 monocytic cells with 27-hydroxycholesterol (27OHChol) resulted in induction of gene transcription of TLR6 and elevated level of cell surface TLR6. Addition of FSL-1, a TLR6 agonist, to 27OHChol-treated cells resulted in transcription of the IL-1alpha gene and enhanced secretion of the corresponding gene product. However, cholesterol did not affect TLR6 expression, and addition of FSL-1 to cholesterol-treated cells did not induce expression of IL-1alpha . Using pharmacological inhibitors, we investigated molecular mechanisms underlying the expression of TLR6 and IL-1alpha. Treatment with Akt inhibitor IV or U0126 resulted in significantly attenuated expression of TLR6 and IL-1alpha induced by 27OHChol and 27OHChol plus FSL-1, respectively. In addition, treatment with LY294002, SB202190, or SP600125 resulted in significantly attenuated secretion of IL-1alpha . These results indicate that 27OHChol can induce inflammation by augmentation of TLR6-mediated production of IL-1alpha in monocytic cells via multiple signaling pathways.
MeSH Terms
Figure
Cited by 2 articles
-
27-Hydroxycholesterol induces macrophage gene expression
via LXR-dependent and -independent mechanisms
Bo-Young Kim, Yonghae Son, Hyok-rae Cho, Dongjun Lee, Seong-Kug Eo, Koanhoi Kim
Korean J Physiol Pharmacol. 2021;25(2):111-118. doi: 10.4196/kjpp.2021.25.2.111.The role of 27-hydroxycholesterol in meta-inflammation
Yonghae Son, Eunbeen Choi, Yujin Hwang, Koanhoi Kim
Korean J Physiol Pharmacol. 2024;28(2):107-112. doi: 10.4196/kjpp.2024.28.2.107.
Reference
-
1. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009; 27:519–550. PMID: 19302047.
Article2. Apte RN, Voronov E. Interleukin-1--a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 2002; 12:277–290. PMID: 12147202.
Article3. Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, Huszar M, White MR, Dinarello CA, Apte RN. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009; 183:4705–4714. PMID: 19752225.
Article4. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996; 87:2095–2147. PMID: 8630372.
Article5. Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999; 145:33–43. PMID: 10428293.
Article6. Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004; 110:1678–1685. PMID: 15353494.7. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34:637–650. PMID: 21616434.
Article9. Curtiss LK, Black AS, Bonnet DJ, Tobias PS. Atherosclerosis induced by endogenous and exogenous toll-like receptor (TLR)1 or TLR6 agonists. J Lipid Res. 2012; 53:2126–2132. PMID: 22822027.
Article10. Schroepfer GJ Jr. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000; 80:361–554. PMID: 10617772.
Article11. Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999; 142:1–28. PMID: 9920502.
Article12. Garcia-Cruset S, Carpenter KL, Guardiola F, Stein BK, Mitchinson MJ. Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic Res. 2001; 35:31–41. PMID: 11697115.
Article13. Vejux A, Kahn E, Dumas D, Bessède G, Ménétrier F, Athias A, Riedinger JM, Frouin F, Stoltz JF, Ogier-Denis E, Todd-Pokropek A, Lizard G. 7-Ketocholesterol favors lipid accumulation and colocalizes with Nile Red positive cytoplasmic structures formed during 7-ketocholesterol-induced apoptosis: analysis by flow cytometry, FRET biphoton spectral imaging microscopy, and subcellular fractionation. Cytometry A. 2005; 64:87–100. PMID: 15739183.14. Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim CD, Kim K. 27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes. Toxicol Appl Pharmacol. 2014; 274:462–470. PMID: 24370436.
Article15. Kim SM, Lee SA, Kim BY, Bae SS, Eo SK, Kim K. 27-Hydroxycholesterol induces recruitment of monocytic cells by enhancing CCL2 production. Biochem Biophys Res Commun. 2013; 442:159–164. PMID: 24269812.
Article16. Prunet C, Montange T, Véjux A, Laubriet A, Rohmer JF, Riedinger JM, Athias A, Lemaire-Ewing S, Néel D, Petit JM, Steinmetz E, Brenot R, Gambert P, Lizard G. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A. 2006; 69:359–373. PMID: 16604541.
Article17. Won K, Kim SM, Lee SA, Rhim BY, Eo SK, Kim K. Multiple signaling molecules are involved in expression of CCL2 and IL-1β in response to FSL-1, a Toll-like receptor 6 agonist, in macrophages. Korean J Physiol Pharmacol. 2012; 16:447–453. PMID: 23271927.
Article18. Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005; 1754:253–262. PMID: 16198162.
Article19. Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Röschmann K, Jung G, Wiesmüller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2008; 83:692–701. PMID: 18056480.
Article20. Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010; 11:155–161. PMID: 20037584.
Article21. Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol. 2007; 297:277–295. PMID: 17466590.
Article22. Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007; 5:264–277. PMID: 17363965.
Article23. Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun. 2004; 72:1657–1665. PMID: 14977973.
Article24. Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012; 221:2–11. PMID: 21978918.
Article25. Carpenter KL, Taylor SE, van der Veen C, Williamson BK, Ballantine JA, Mitchinson MJ. Lipids and oxidised lipids in human atherosclerotic lesions at different stages of development. Biochim Biophys Acta. 1995; 1256:141–150. PMID: 7766691.
Article26. Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006; 103:2274–2279. PMID: 16461893.
Article27. Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J Cell Physiol. 2007; 210:667–675. PMID: 17117477.
Article28. Chen X, Resh MD. Activation of mitogen-activated protein kinase by membrane-targeted Raf chimeras is independent of raft localization. J Biol Chem. 2001; 276:34617–34623. PMID: 11457834.
Article29. El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J. 2003; 17:720–722. PMID: 12586735.30. Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994; 54:2419–2423. PMID: 8162590.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 7alpha-Hydroxycholesterol Elicits TLR6-Mediated Expression of IL-23 in Monocytic Cells
- Multiple Signaling Molecules are Involved in Expression of CCL2 and IL-1beta in Response to FSL-1, a Toll-Like Receptor 6 Agonist, in Macrophages
- Expression of Toll-like Receptor 4 on Human Keratinocytes by Lipoteichoic Acid
- Peptidoglycan Induces the Production of Interleukin-8 via Calcium Signaling in Human Gingival Epithelium
- A Study of Production of Interleukin-1alpha by Peripheral Lymphocytes in Patients with Coronary Artery Disease