J Bacteriol Virol.
2012 Jun;42(2):121-132. 10.4167/jbv.2012.42.2.121.
Intracellular Signaling Pathways that Regulate Macrophage Chemokine Expression in Response to Mycobacterium abscessus
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Daejeon, Korea. hayoungj@cnu.ac.kr
- 2Infection Signaling Network Research Center, College of Medicine, Chungnam National University, Daejeon, Korea.
- 3Department of Research and Development, Korean Institute of Tuberculosis, Osong Bio-Health Science Technopolis, Chungbuk, Korea.
- KMID: 1717683
- DOI: http://doi.org/10.4167/jbv.2012.42.2.121
Abstract
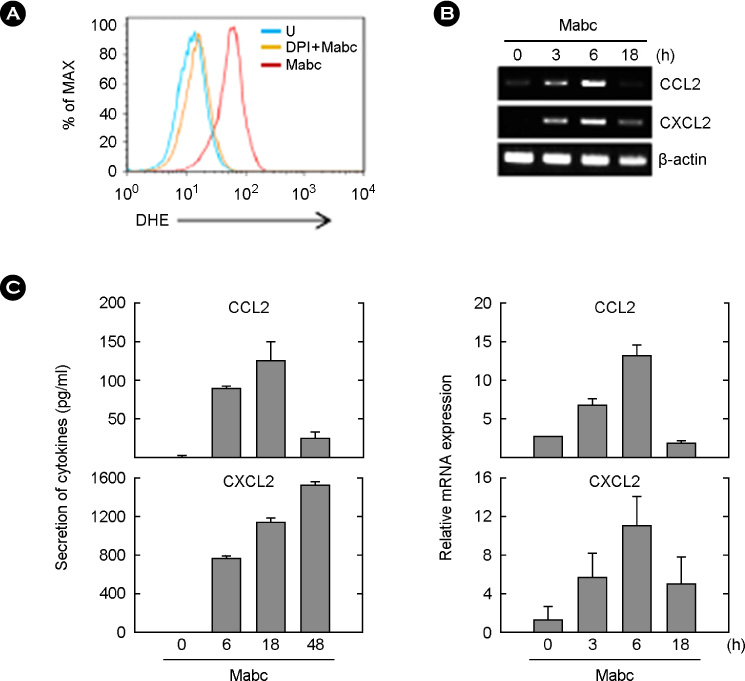
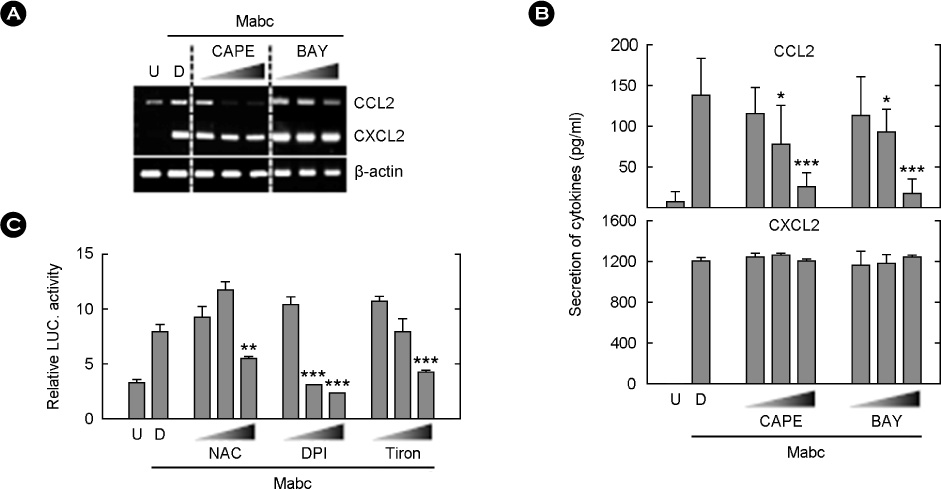
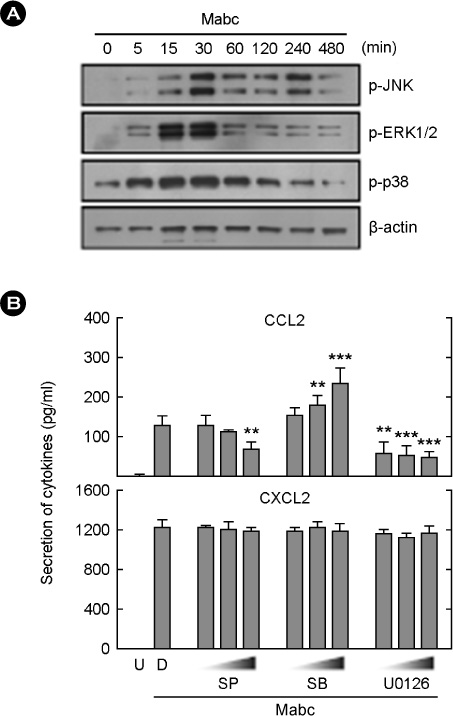
- Mycobacterium abscessus (Mabc) is an emerging human pathogen. Less is known about the host immune response to Mabc than to M. tuberculosis. Here, we examined the intracellular signaling pathways that govern the expression of chemokines including (C-C motif) ligand 2 (CCL2) and (C-X-C motif) ligand 2 (CXCL2) in macrophages after infection with Mabc. Specifically, Mabc triggered the generation of reactive oxygen species (ROS) and the production of CCL2 and CXCL2 in murine bone marrow-derived macrophages (BMDMs). Mabc-induced CCL2, but not CXCL2, was dependent on the generation of ROS. Toll-like receptor (TLR) 2, MyD88, but not TRIF, was required for Mabc-induced CCL2 and CXCL2 expression. Additionally, Mabc infection significantly induced nuclear factor (NF)-kappaB nuclear translocation and luciferase activity. The activation of NF-kappaB was required for Mabc-induced CCL2, but not CXCL2 expression. Moreover, Mabc-induced ROS generation was required for NF-kappaB activation. Treatment of BMDMs with Mabc rapidly induced the activation of mitogen-activated protein kinase (MAPKs) pathways. Interestingly, CCL2 expression was dependent on the activation of JNK and ERK1/2 pathways, whereas it was negatively regulated by the p38 MAPK pathway. In contrast, Mabc-dependent CXCL2 expression was not regulated by MAPK pathways. These data suggest that intracellular ROS generation is required for innate and inflammatory responses during Mabc infection of macrophages.
Keyword
MeSH Terms
Figure
Reference
-
1. Medjahed H, Gaillard JL, Reyrat JM. Mycobacterium abscessus: a new player in the mycobacterial field. Trends Microbiol. 2010. 18:117–123.2. Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS. 2006. 114:319–328.
Article3. Huynh KK, Joshi SA, Brown EJ. A delicate dance: host response to mycobacteria. Curr Opin Immunol. 2011. 23:464–472.
Article4. Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008. 21:279–286.
Article5. Bachmann MF, Kopf M, Marsland BJ. Chemokines: more than just road signs. Nat Rev Immunol. 2006. 6:159–164.
Article6. Natarajan K, Kundu M, Sharma P, Basu J. Innate immune responses to M. tuberculosis infection. Tuberculosis (Edinb). 2011. 91:427–431.7. Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006. 72:1102–1113.
Article8. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007. 9:1087–1098.
Article9. Yuk JM, Jo EK. Toll-like Receptors and Innate Immunity. J Bacteriol Virol. 2011. 41:225–235.
Article10. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011. 2011:405310.11. Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000. 12:64–76.
Article12. Kohchi C, Inagawa H, Nishizawa T, Soma G. ROS and innate immunity. Anticancer Res. 2009. 29:817–821.13. Steevels TA, Meyaard L. Immune inhibitory receptors: essential regulators of phagocyte function. Eur J Immunol. 2011. 41:575–587.
Article14. Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011. 32:491–509.
Article15. Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009. 182:3696–3705.
Article16. Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008. 10:1608–1621.
Article17. Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005. 1754:253–262.
Article18. Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, et al. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 2007. 9:382–396.
Article19. Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011. 195:1083–1092.
Article20. Lee HM, Yuk JM, Shin DM, Yang CS, Kim KK, Choi DK, et al. Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J Immunol. 2009. 183:6839–6848.
Article21. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010. 11:373–384.
Article22. Kim E, Kim SH, Kim S, Kim TS. The novel cytokine p43 induces IL-12 production in macrophages via NF-kappaB activation, leading to enhanced IFN-gamma production in CD4+ T cells. J Immunol. 2006. 176:256–264.
Article23. Jo EK, Park JK, Dockrell HM. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr Opin Infect Dis. 2003. 16:205–210.
Article24. Lee JS, Lee JY, Son JW, Oh JH, Shin DM, Yuk JM, et al. Expression and regulation of the CC-chemokine ligand 20 during human tuberculosis. Scand J Immunol. 2008. 67:77–85.
Article25. Elkington PT, D'Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med. 2011. 3:71ps6.
Article26. Kang DD, Lin Y, Moreno JR, Randall TD, Khader SA. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One. 2011. 6:e16161.
Article27. Kipnis A, Basaraba RJ, Orme IM, Cooper AM. Role of chemokine ligand 2 in the protective response to early murine pulmonary tuberculosis. Immunology. 2003. 109:547–551.
Article28. Jeong E, Lee JY. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med J. 2011. 52:379–392.
Article29. Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999. 96:14459–14463.
Article30. Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007. 179:1178–1189.
Article31. Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999. 31:53–59.
Article32. Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, et al. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004. 24:4384–4394.
Article33. Kwon MJ, Han J, Kim BH, Lee YS, Kim TY. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: superoxide dismutase 3 inhibits reactive oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid Redox Signal. 2012. 16:297–313.
Article34. Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFkappaB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008. 1780:973–982.
Article35. Lee HM, Shin DM, Choi DK, Lee ZW, Kim KH, Yuk JM, et al. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell Microbiol. 2009. 11:678–692.
Article36. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003. 5:133–142.
Article37. Sampaio EP, Elloumi HZ, Zelazny A, Ding L, Paulson ML, Sher A, et al. Mycobacterium abscessus and M. avium trigger Toll-like receptor 2 and distinct cytokine response in human cells. Am J Respir Cell Mol Biol. 2008. 39:431–439.
Article38. Bai X, Chmura K, Ovrutsky AR, Bowler RP, Scheinman RI, Oberley-Deegan RE, et al. Mycobacterium tuberculosis increases IP-10 and MIG protein despite inhibition of IP-10 and MIG transcription. Tuberculosis (Edinb). 2011. 91:26–35.
Article