Ann Dermatol.
2015 Apr;27(2):142-151. 10.5021/ad.2015.27.2.142.
Ethanol Extract of Peanut Sprout Exhibits a Potent Anti-Inflammatory Activity in Both an Oxazolone-Induced Contact Dermatitis Mouse Model and Compound 48/80-Treated HaCaT Cells
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. schul@jnu.ac.kr
- 2Department of Urology, Chonnam National University Medical School, Gwangju, Korea.
- 3Jangsoochae Company, Chuncheon, Korea.
- KMID: 2264802
- DOI: http://doi.org/10.5021/ad.2015.27.2.142
Abstract
- BACKGROUND
We developed an ethanol extract of peanut sprouts (EPS), a peanut sprout-derived natural product, which contains a high level of trans-resveratrol (176.75 microg/ml) and was shown to have potent antioxidant activity.
OBJECTIVE
We evaluated the potential anti-inflammatory activity of EPS by measuring its antioxidant potential in skin.
METHODS
The anti-inflammatory activity of EPS was tested using two models of skin inflammation: oxazolone (OX)-induced contact dermatitis in mice and compound 48/80-treated HaCaT cells. As biomarkers of skin inflammation, cyclooxygenase-2 (COX-2) and nerve growth factor (NGF) levels were measured.
RESULTS
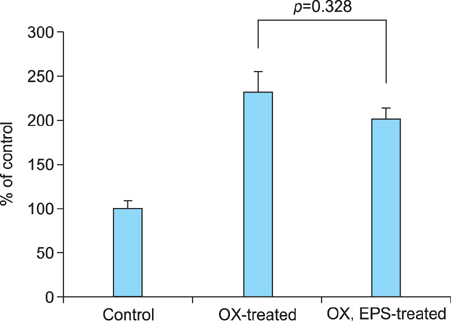
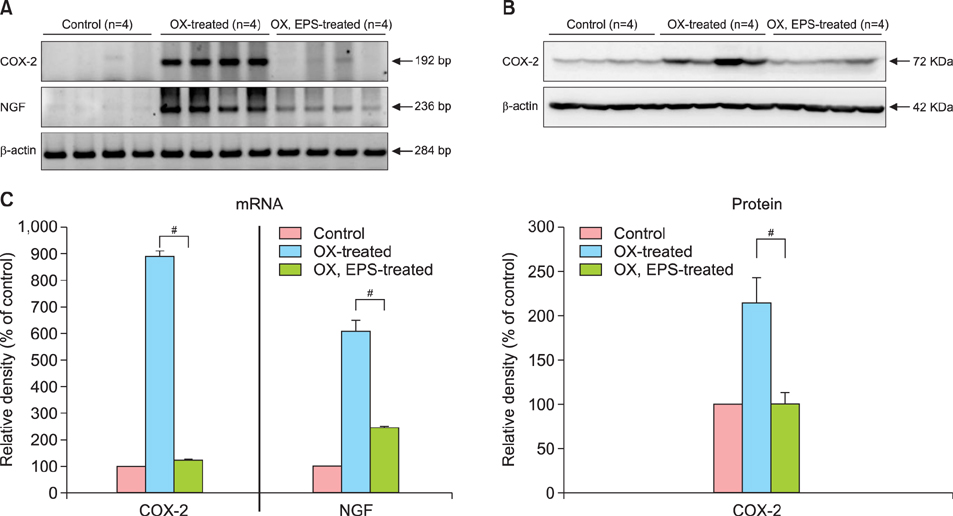
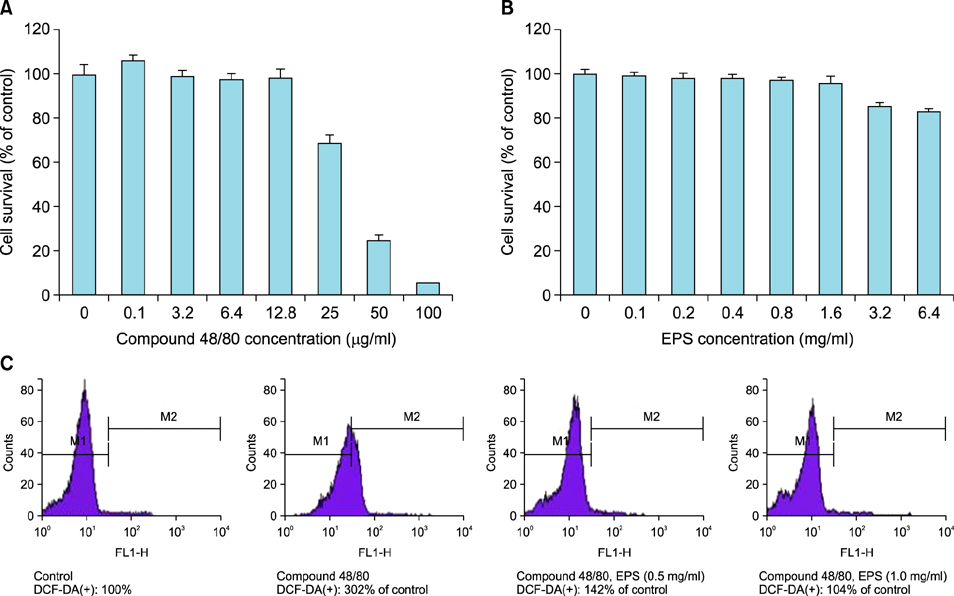
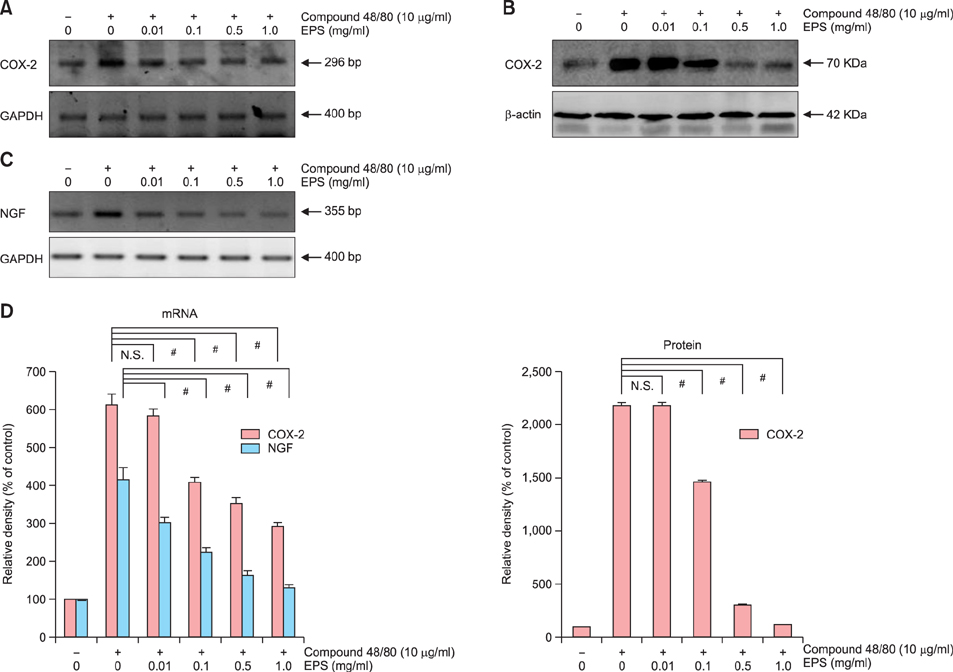
OX-induced contact dermatitis was suppressed markedly in mice that were treated with an ointment containing 5% EPS as evidenced by a decrease in the extent of scaling and thickening (p<0.05) and supported by a histological study. COX-2 (messenger RNA [mRNA] and protein) and NGF (mRNA) levels, which were upregulated in the skin of OX-treated mice, were suppressed markedly in the skin of OX+EPS-treated mice. Consistent with this, compound 48/80-induced expression of COX-2 (mRNA and protein) and NGF (mRNA) in HaCaT cells were suppressed by EPS treatment in a dose-dependent manner. As an inhibitor of NF-kappaB, IkappaB protein levels were dose-dependently upregulated by EPS. Fluorescence-activated cell sorting (FACS) analysis revealed that EPS scavenged compound 48/80-induced reactive oxygen species (ROS) in HaCaT cells.
CONCLUSION
EPS exerts a potent anti-inflammatory activity via its anti-oxidant activity in both mouse skin and compound 48/80-treated HaCaT cells in vitro. Compound 48/80-treated HaCaT cells are a useful new in vitro model of skin inflammation.
Keyword
MeSH Terms
-
Animals
Biomarkers
Cyclooxygenase 2
Dermatitis, Contact*
Ethanol*
Flow Cytometry
Inflammation
Mice*
Nerve Growth Factor
NF-kappa B
Oxazolone
p-Methoxy-N-methylphenethylamine
Reactive Oxygen Species
RNA
Skin
Cyclooxygenase 2
Ethanol
NF-kappa B
Nerve Growth Factor
Oxazolone
RNA
Reactive Oxygen Species
p-Methoxy-N-methylphenethylamine
Figure
Cited by 1 articles
-
CSP0510 Lotion as a Novel Moisturizer Containing Citric Acid and Trisodium Phosphate Relieves Objective and Subjective Symptoms of Atopic Dermatitis
Ho-June Lee, Nam-Woong Yang, Jee-Young Choi, Jee-Bum Lee, Seung-Chul Lee
Ann Dermatol. 2016;28(3):344-351. doi: 10.5021/ad.2016.28.3.344.
Reference
-
1. Sales JM, Resurreccion AV. Resveratrol in peanuts. Crit Rev Food Sci Nutr. 2014; 54:734–770.
Article2. Choi JY, Choi DI, Lee JB, Yun SJ, Lee DH, Eun JB, et al. Ethanol extract of peanut sprout induces Nrf2 activation and expression of antioxidant and detoxifying enzymes in human dermal fibroblasts: implication for its protection against UVB-irradiated oxidative stress. Photochem Photobiol. 2013; 89:453–460.
Article3. Gallicchio M, Rosa AC, Benetti E, Collino M, Dianzani C, Fantozzi R. Substance P-induced cyclooxygenase-2 expression in human umbilical vein endothelial cells. Br J Pharmacol. 2006; 147:681–689.
Article4. Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993; 262:1569–1572.
Article5. Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002; 23:1507–1515.
Article6. Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005; 4:517–519.
Article7. Truzzi F, Marconi A, Pincelli C. Neurotrophins in healthy and diseased skin. Dermatoendocrinol. 2011; 3:32–36.
Article8. Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011; 15:33–39.
Article9. Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009; 53:48–54.
Article10. Lee JH, Piao MS, Choi JY, Yun SJ, Lee JB, Lee SC. Up-regulation of cyclooxygenase 2 and matrix metalloproteinases-2 and -9 in cutaneous squamous cell carcinoma: active role of inflammation and tissue remodeling in carcinogenesis. Ann Dermatol. 2013; 25:145–151.
Article11. Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003; 192:294–306.
Article12. Anggakusuma , Yanti , Hwang JK. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J Dermatol Sci. 2010; 57:114–122.
Article13. Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008; 128:79–86.
Article14. Portugal M, Barak V, Ginsburg I, Kohen R. Interplay among oxidants, antioxidants, and cytokines in skin disorders: present status and future considerations. Biomed Pharmacother. 2007; 61:412–422.
Article15. Pascual-Martí MC, Salvador A, Chafer A, Berna A. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta. 2001; 54:735–740.
Article16. Paulo L, Domingues F, Queiroz JA, Gallardo E. Development and validation of an analytical method for the determination of trans- and cis-resveratrol in wine: analysis of its contents in 186 Portuguese red wines. J Agric Food Chem. 2011; 59:2157–2168.
Article17. Kang HI, Kim JY, Kwon SJ, Park KW, Kang JS, Seo KI. Antioxidative effects of peanut sprout extracts. J Korean Soc Food Sci Nutr. 2010; 39:941–946.
Article18. Kim HJ, Kang JS, Park HR, Hwang YI. Neuroprotective effects of methanolic extracts from peanut sprouts. J Life Sci. 2010; 20:253–259.
Article19. Wang KH, Lai YH, Chang JC, Ko TF, Shyu SL, Chiou RY. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J Agric Food Chem. 2005; 53:242–246.
Article20. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006; 5:493–506.
Article21. Hung CF, Lin YK, Huang ZR, Fang JY. Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol Pharm Bull. 2008; 31:955–962.
Article22. Le Filliatre G, Sayah S, Latournerie V, Renaud JF, Finet M, Hanf R. Cyclo-oxygenase and lipoxygenase pathways in mast cell dependent-neurogenic inflammation induced by electrical stimulation of the rat saphenous nerve. Br J Pharmacol. 2001; 132:1581–1589.
Article23. Tominaga M, Takamori K. An update on peripheral mechanisms and treatments of itch. Biol Pharm Bull. 2013; 36:1241–1247.
Article24. Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994; 14:1422–1432.
Article25. Kou K, Nakamura F, Aihara M, Chen H, Seto K, Komori-Yamaguchi J, et al. Decreased expression of semaphorin-3A, a neurite-collapsing factor, is associated with itch in psoriatic skin. Acta Derm Venereol. 2012; 92:521–528.
Article26. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003; 149:718–730.
Article27. Santambrogio L, Benedetti M, Chao MV, Muzaffar R, Kulig K, Gabellini N, et al. Nerve growth factor production by lymphocytes. J Immunol. 1994; 153:4488–4495.28. Shi Y, Jin Y, Guo W, Chen L, Liu C, Lv X. Blockage of nerve growth factor modulates T cell responses and inhibits allergic inflammation in a mouse model of asthma. Inflamm Res. 2012; 61:1369–1378.
Article29. Jallat-Daloz I, Cognard JL, Badet JM, Regnard J. Neuralepithelial cell interplay: in vitro evidence that vagal mediators increase PGE2 production by human nasal epithelial cells. Allergy Asthma Proc. 2001; 22:17–23.
Article30. Song IS, Bunnett NW, Olerud JE, Harten B, Steinhoff M, Brown JR, et al. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R). Exp Dermatol. 2000; 9:42–52.
Article31. Brooks AC, Whelan CJ, Purcell WM. Reactive oxygen species generation and histamine release by activated mast cells: modulation by nitric oxide synthase inhibition. Br J Pharmacol. 1999; 128:585–590.
Article32. Baolin L, Weiwei W, Ning T. Topical application of luteolin inhibits scratching behavior associated with allergic cutaneous reaction in mice. Planta Med. 2005; 71:424–428.
Article33. Takubo M, Ueda Y, Yatsuzuka R, Jiang S, Fujii Y, Kamei C. Characteristics of scratching behavior induced by some chemical mediators in hairless mice. J Pharmacol Sci. 2006; 100:285–288.
Article34. Ikai K, Danno K, Horio T, Narumiya S. Effect of ultraviolet irradiation on mast cell-deficient W/Wv mice. J Invest Dermatol. 1985; 85:82–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Particulate Matter in a Mouse Model of Oxazolone-Induced Atopic Dermatitis
- Dioscorea japonica Thunb. Ethanolic Extract Attenuated Oxazolone-Induced Atopic Dermatitis-like Skin Lesions in BALB/c Mice
- Inhibitory Effects of CD99-derived Peptide CD99CRIII3 on the Extravasation of Monocytes and Inflammatory Reactions in Contact Dermatitis Mouse Model
- Peanut sprout ethanol extract inhibits the adipocyte proliferation, differentiation, and matrix metalloproteinases activities in mouse fibroblast 3T3-L1 preadipocytes
- Ethanol Extract of Chaenomeles sinensis Inhibits the Development of Benign Prostatic Hyperplasia by Exhibiting Anti-oxidant and Anti-inflammatory Effects