Allergy Asthma Immunol Res.
2010 Jan;2(1):34-40. 10.4168/aair.2010.2.1.34.
Preventive effects of mycobacteria and their culture supernatants against asthma development in BALB/c mice
- Affiliations
-
- 1Department of Allergy, Chonnam National University Medical School, Gwangju, Korea. ischoi@chonnam.ac.kr
- 2Department of Microbiology, College of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 2260417
- DOI: http://doi.org/10.4168/aair.2010.2.1.34
Abstract
- PURPOSE
Live Mycobacterium bovis Bacille Calmette-Guerin (BCG) has a suppressive effect on asthma, but its use in clinical practice may be limited due to adverse reactions. To develop a product that is effective for suppressing asthma with minimal adverse reactions, we investigated whether the heat-killed body or culture supernatants of mycobacteria could also prevent asthma development.
METHODS
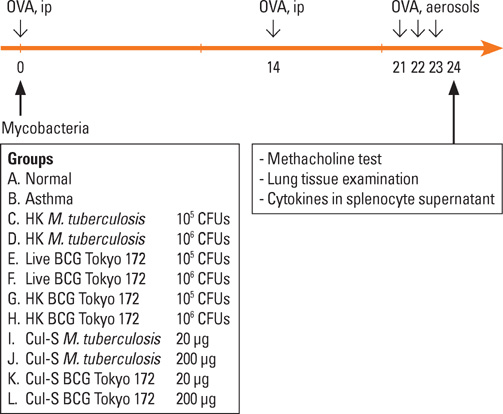
Female BALB/c mice were treated with live BCG, the heat-killed body, or culture supernatants of BCG or Mycobacterium tuberculosis intraperitoneally, while sensitizing and provoking with ovalbumin. Then they underwent a methacholine bronchoprovocation test, and the peribronchial inflammatory cell numbers and cytokine levels in splenocyte culture supernatants were assessed.
RESULTS
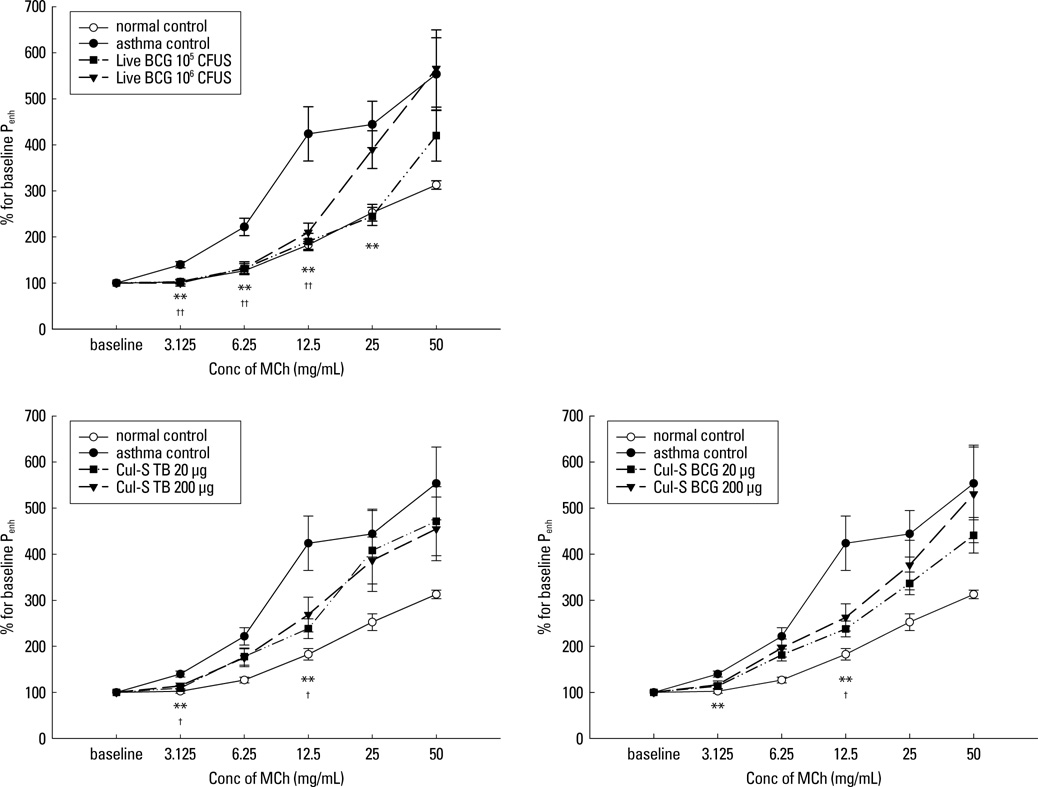
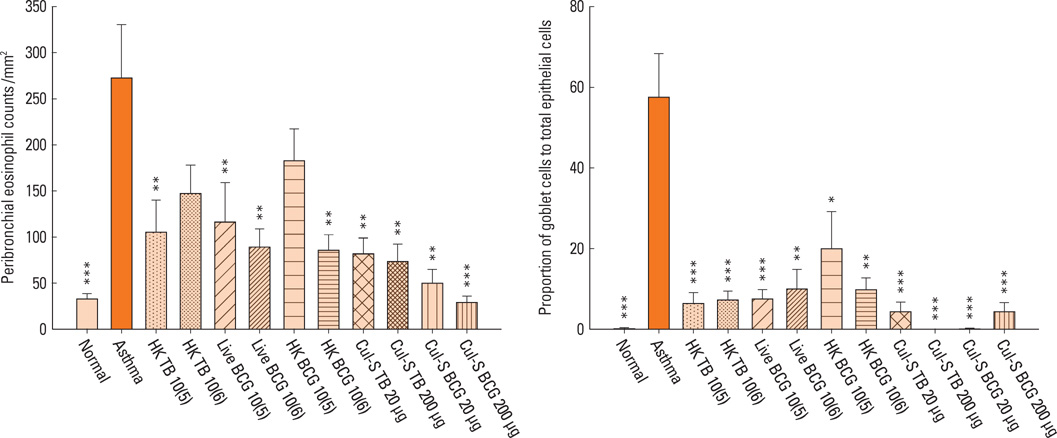
The airway sensitivity to methacholine decreased significantly after treatment with not only live BCG (30.8 versus 10.0 mg/mL, P<0.001) but also with the culture supernatant (BCG, 23.0 mg/mL, P<0.05; M. tuberculosis, 20.5 mg/mL, P<0.05). In contrast, heat-killed mycobacteria did not effectively decrease airway sensitivity. The peribronchial eosinophil counts and the goblet cell proportions in total epithelial cells decreased significantly in most of the groups. The interferon-gamma/interleukin-5 ratios increased significantly in most of the treatment groups except for the heat-killed groups, and were significantly related to airway sensitivity (r=0.312, P<0.01) and peribronchial eosinophil counts (r=-0.416, P<0.001). Interleukin-17A level was inversely related to airway sensitivity (r=-0.212, P<0.05) and was significantly lower in the live BCG group than in the control (137+/-20 versus 308+/-57 pg/mL, P<0.05).
CONCLUSIONS
BCG and mycobacteria culture supernatants may effectively prevent the development of asthma associated with altered Th1/Th2 cytokines and interleukin-17A levels.
Keyword
MeSH Terms
-
Animals
Asthma
BCG Vaccine
Cell Count
Cytokines
Eosinophils
Epithelial Cells
Female
Goblet Cells
Humans
Interferons
Interleukin-17
Interleukins
Methacholine Chloride
Mice
Mycobacterium
Mycobacterium bovis
Mycobacterium tuberculosis
Ovalbumin
Tuberculosis
BCG Vaccine
Cytokines
Interferons
Interleukin-17
Interleukins
Methacholine Chloride
Ovalbumin
Figure
Cited by 1 articles
-
Therapeutic Effects of Mycobacterial Secretory Proteins Against Established Asthma in BALB/c Mice
Eui-Ryoung Han, Inseon S. Choi, Han-Gyu Choi, Hwa-Jung Kim
Allergy Asthma Immunol Res. 2012;4(4):214-221. doi: 10.4168/aair.2012.4.4.214.
Reference
-
1. Rook GAW, Hamelmann EH, Brunet LR. Mycobacteria and allergies. Immunobiology. 2007. 212:461–473.2. Koh YI, Choi IS, Kim WY. BCG infection in allergen-presensitized rats suppresses Th2 immune response and prevents the development of allergic asthmatic reaction. J Clin Immunol. 2001. 21:51–59.3. Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998. 187:561–569.4. Herz U, Gerhold K, Grüber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998. 102:867–874.5. Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998. 93:307–313.6. Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol. 2002. 168:2516–2522.7. Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002. 8:625–629.8. Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002. 88:584–591.9. Shirtcliffe PM, Easthope SE, Weatherall M, Beasley R. Effect of repeated intradermal injections of heat-inactivated Mycobacterium bovis bacillus Calmette-Guérin in adult asthma. Clin Exp Allergy. 2004. 34:207–212.10. Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005. 95:571–578.11. Choi IS, Lin XH, Koh YA, Cui Y. Inoculation route-dependent and allergen-specific suppressive effects of bacille Calmette-Guérin vaccination on asthmatic reactions in BALB/c mice. Lung. 2007. 185:179–186.12. Barlan I, Bahceciler NN, Akdis M, Akdis CA. Bacillus Calmette-Guérin, Mycobacterium bovis, as an immunomodulator in atopic diseases. Immunol Allergy Clin North Am. 2006. 26:365–377.13. Sayers I, Severn W, Scanga CB, Hudson J, Le Gros G, Harper JL. Suppression of allergic airway disease using mycobacterial lipoglycans. J Allergy Clin Immunol. 2004. 114:302–309.14. Ito T, Hasegawa A, Hosokawa H, Yamashita M, Motohashi S, Naka T, Okamoto Y, Fujita Y, Ishii Y, Taniguchi M, Yano I, Nakayama T. Human Th1 differentiation induced by lipoarabinomannan/lipomannan from Mycobacterium bovis BCG Tokyo-172. Int Immunol. 2008. 20:849–860.15. Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, Garcia VE, Krutzik SR, Weis SE, Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008. 38:459–469.16. Akdis CA, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, Klunker S, Isitmangil G, Hansjee N, Wynn TA, Dillon S, Erb P, Baschang G, Blaser K, Alkan SS. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003. 33:2717–2726.17. Riffo-Vasquez Y, Spina D, Page C, Tormay P, Singh M, Henderson B, Coates A. Effect of Mycobacterium tuberculosis chaperonins on bronchial eosinophilia and hyper-responsiveness in a murine model of allergic inflammation. Clin Exp Allergy. 2004. 34:712–719.18. Ozdemir C, Akkoc T, Bahceciler NN, Kucukercan D, Barlan IB, Basaran MM. Impact of Mycobacterium vaccae immunization on lung histopathology in a murine model of chronic asthma. Clin Exp Allergy. 2003. 33:266–270.19. Maestrelli P, Saetta M, Di Stefano A, Calcagni PG, Turato G, Ruggieri MP, Roggeri A, Mapp CE, Fabbri LM. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med. 1995. 152:1926–1931.20. Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, Takahashi Y, Nagai H. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res. 2001. 50:616–624.21. Yang X, Wang S, Fan Y, Zhu L. Systemic mycobacterial infection inhibits antigen-specific immunoglobulin E production, bronchial mucus production and eosinophilic inflammation induced by allergen. Immunology. 1999. 98:329–337.22. Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999. 163:3920–3927.23. Major T Jr, Wohlleben G, Reibetanz B, Erb KJ. Application of heat killed Mycobacterium bovis-BCG into the lung inhibits the development of allergen-induced Th2 responses. Vaccine. 2002. 20:1532–1540.24. Chernousova LN, Smirnova TG, Afanasieva EG, Karpov VL, Timofeev AV. Ex vivo production of interferon-γ, tumor necrosis factor-α, and interleukin-6 by mouse macrophages during infection with M. bovis and M. tuberculosis H37Rv. Bull Exp Biol Med. 2007. 144:709–712.25. von Mutius E, Pearce N, Beasley R, Cheng S, von Ehrenstein O, Bjorksten B, Weiland S. International patterns of tuberculosis and the prevalence of symptoms of asthma, rhinitis, and eczema. Thorax. 2000. 55:449–453.26. Grüber C, Meinlschmidt G, Bergmann R, Wahn U, Stark K. Is early BCG vaccination associated with less atopic disease? An epidemiological study in German preschool children with different ethnic backgrounds. Pediatr Allergy Immunol. 2002. 13:177–181.27. Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003. 28:42–50.28. McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008. 181:4089–4097.29. Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007. 120:247–254.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effects of Mycobacterial Secretory Proteins Against Established Asthma in BALB/c Mice
- Viscerotropic growth pattern of Leishmania tropica in BALB/c mice is suggestive of a murine model for human viscerotropic leishmaniasis
- Immunomodulation Therapy using Mycobacteria in Asthma
- Immunomodulating Approach to Asthma Using Mycobacteria
- Preventive Effect of Combined Administration of BCG and DHEA against Development of Asthma