Allergy Asthma Immunol Res.
2012 Jul;4(4):214-221. 10.4168/aair.2012.4.4.214.
Therapeutic Effects of Mycobacterial Secretory Proteins Against Established Asthma in BALB/c Mice
- Affiliations
-
- 1Department of Allergy, Chonnam National University Medical School, Gwangju, Korea. ischoi@chonnam.ac.kr
- 2Department of Microbiology and Research Institute for Medical Science, College of Medicine, Chungnam National University, Daejeon, Korea. hjukim@cnu.ac.kr
- KMID: 1970710
- DOI: http://doi.org/10.4168/aair.2012.4.4.214
Abstract
- PURPOSE
Live/killed mycobacteria and culture supernatants can suppress asthmatic reactions. This study investigated whether mycobacterial secretory proteins have therapeutic effects on asthma.
METHODS
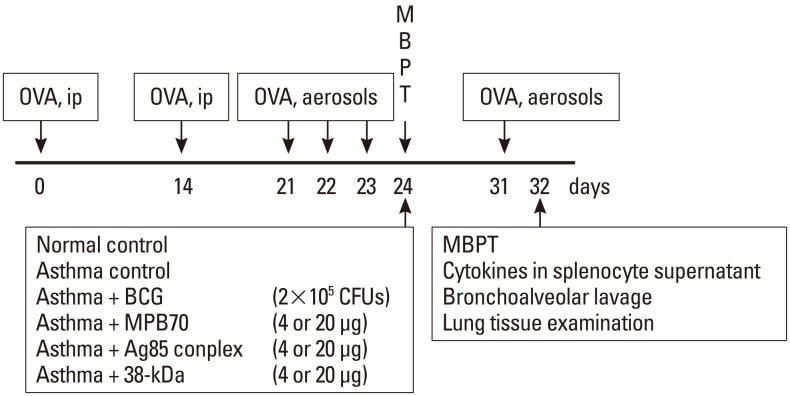
Mycobacterium bovis bacille Calmette-Guerin (BCG; 2x105 CFUs) and mycobacterial secretory proteins (Ag85 complex, 38-kDa protein or MPB70; 4 or 20 microg) were administered intraperitoneally to female BALB/c mice with established airway hyperresponsiveness. One week after treatment, the mice underwent a methacholine challenge test, and then inflammatory cell numbers in bronchoalveolar lavage fluid (BAL) and around bronchi (<500 microm), and cytokine levels in splenocyte supernatants, were assessed.
RESULTS
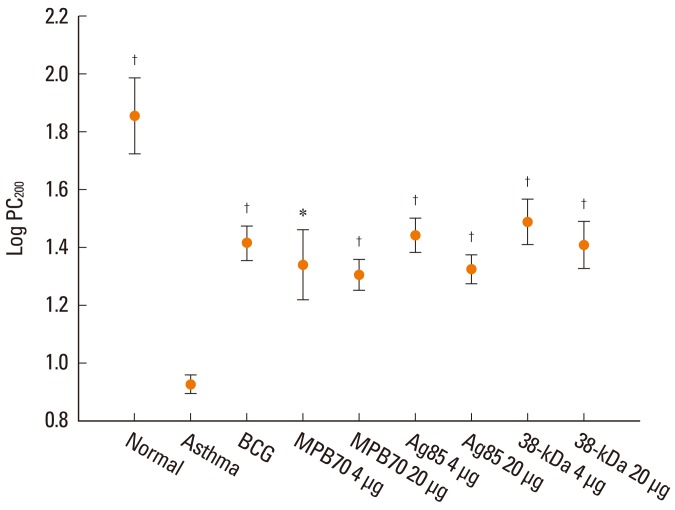
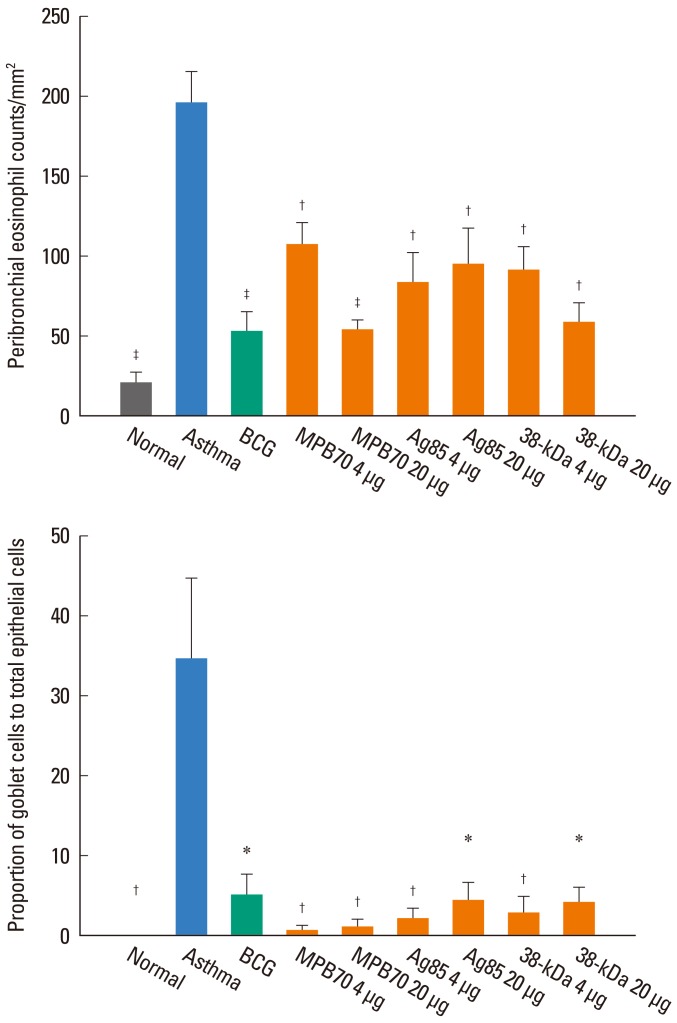
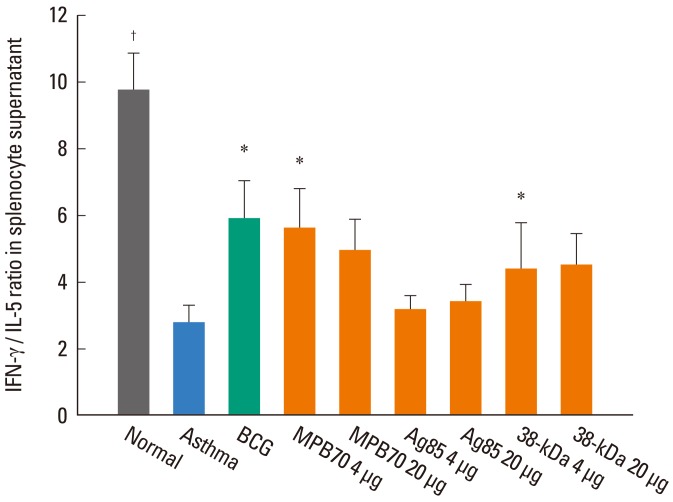
BCG and all of the tested secretory proteins significantly improved airway sensitivity compared to baseline values (P<0.05). The secretory protein Ag85 complex significantly suppressed airway reactivity also (P<0.05), while 38-kDa protein significantly suppressed reactivity and maximal narrowing (P<0.05). The number of eosinophils in BAL and around bronchi, and the goblet cell proportion, were also significantly reduced in mice in both the BCG and secretory protein groups compared to the asthma control group. IFN-gamma/IL-5 ratios were significantly higher in mice treated with BCG, 4 microg MPB70 or 4 microg 38-kDa protein than in asthma control mice (P<0.05), and were negatively associated with airway hyperresponsiveness, peribronchial eosinophil numbers and goblet cell proportion (all P<0.05). IL-17A was positively correlated with IL-5 (r=0.379, P<0.001), maximal airway narrowing, peribronchial eosinophil numbers and goblet cell proportion (all P<0.05).
CONCLUSIONS
Secretory proteins from BCG and M. tuberculosis and live BCG were effective against established asthma, their effects being accompanied by increased IFN-gamma/IL-5 ratios. Thus, allergic asthma could be effectively treated with mycobacterial secretory proteins.
Keyword
MeSH Terms
Figure
Reference
-
1. Ninan TK, Russell G. Respiratory symptoms and atopy in Aberdeen schoolchildren: evidence from two surveys 25 years apart. BMJ. 1992; 304:873–875. PMID: 1392746.
Article2. Malik G, Tagiyeva N, Aucott L, McNeill G, Turner SW. Changing trends in asthma in 9-12 year olds between 1964 and 2009. Arch Dis Child. 2011; 96:227–231. PMID: 21068081.
Article3. Annesi-Maesano I, Mourad C, Daures JP, Kalaboka S, Godard P. Time trends in prevalence and severity of childhood asthma and allergies from 1995 to 2002 in France. Allergy. 2009; 64:798–800. PMID: 19183165.
Article4. Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergol Immunopathol (Madr). 2010; 38:83–87. PMID: 20106581.
Article5. Cookson WO, Moffatt MF. Asthma: an epidemic in the absence of infection? Science. 1997; 275:41–42. PMID: 8999535.6. Rook GA, Hamelmann E, Brunet LR. Mycobacteria and allergies. Immunobiology. 2007; 212:461–473. PMID: 17544831.
Article7. Koh YI, Choi IS, Kim WY. BCG infection in allergen-presensitized rats suppresses Th2 immune response and prevents the development of allergic asthmatic reaction. J Clin Immunol. 2001; 21:51–59. PMID: 11321239.8. Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998; 187:561–569. PMID: 9463406.
Article9. Herz U, Gerhold K, Grüber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998; 102:867–874. PMID: 9819307.
Article10. Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998; 93:307–313. PMID: 9640239.
Article11. Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol. 2002; 168:2516–2522. PMID: 11859146.
Article12. Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002; 8:625–629. PMID: 12042815.
Article13. Han ER, Choi IS, Eom SH, Kim HJ. Preventive effects of mycobacteria and their culture supernatants against asthma development in BALB/c mice. Allergy Asthma Immunol Res. 2010; 2:34–40. PMID: 20224676.
Article14. Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992; 56:648–661. PMID: 1480113.
Article15. Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, Song CH, Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006; 74:2686–2696. PMID: 16622205.
Article16. Fonseca DP, Benaissa-Trouw B, van Engelen M, Kraaijeveld CA, Snippe H, Verheul AF. Induction of cell-mediated immunity against Mycobacterium tuberculosis using DNA vaccines encoding cytotoxic and helper T-cell epitopes of the 38-kilodalton protein. Infect Immun. 2001; 69:4839–4845. PMID: 11447158.17. Al-Attiyah R, Shaban FA, Wiker HG, Oftung F, Mustafa AS. Synthetic peptides identify promiscuous human Th1 cell epitopes of the secreted mycobacterial antigen MPB70. Infect Immun. 2003; 71:1953–1960. PMID: 12654813.
Article18. Lin XH, Choi IS, Koh YA, Cui Y. Effects of combined bacille Calmette-Guérin and dehydroepiandrosterone treatment on established asthma in mice. Exp Lung Res. 2009; 35:250–261. PMID: 19337907.
Article19. Lim JH, Park JK, Jo EK, Song CH, Min D, Song YJ, Kim HJ. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect Immun. 1999; 67:6187–6190. PMID: 10531287.20. Lee JS, Paik TH, Yoo YC, Lee J, Shin A, Song CH, Jo EK, Kim HJ, Park JK. Purification of native Ag85 complex, 38-kDa and MTB12 protein antigens from the culture filtrate of Mycobacterium tuberculosis. J Bacteriol Virol. 2006; 36:211–220.21. Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005; 95:571–578. PMID: 16400898.
Article22. Malo JL, Chan-Yeung M. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Occupational asthma. Middleton's Allergy Principles & Practice. 2003. 6th ed. Philadelphia: Mosby Inc.;p. 1333–1352.
Article23. Ozdemir C, Akkoc T, Bahceciler NN, Kucukercan D, Barlan IB, Basaran MM. Impact of Mycobacterium vaccae immunization on lung histopathology in a murine model of chronic asthma. Clin Exp Allergy. 2003; 33:266–270. PMID: 12580921.
Article24. Maestrelli P, Saetta M, Di Stefano A, Calcagni PG, Turato G, Ruggieri MP, Roggeri A, Mapp CE, Fabbri LM. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med. 1995; 152:1926–1931. PMID: 8520757.
Article25. Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, Takahashi Y, Nagai H. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res. 2001; 50:616–624. PMID: 11822788.
Article26. Choi IS, Lin XH, Koh YA, Cui Y. Inoculation route-dependent and allergen-specific suppressive effects of bacille Calmette-Guérin vaccination on asthmatic reactions in BALB/c mice. Lung. 2007; 185:179–186. PMID: 17406942.
Article27. Shirtcliffe PM, Easthope SE, Weatherall M, Beasley R. Effect of repeated intradermal injections of heat-inactivated Mycobacterium bovis bacillus Calmette-Guérin in adult asthma. Clin Exp Allergy. 2004; 34:207–212. PMID: 14987299.28. Sayers I, Severn W, Scanga CB, Hudson J, Le Gros G, Harper JL. Suppression of allergic airway disease using mycobacterial lipoglycans. J Allergy Clin Immunol. 2004; 114:302–309. PMID: 15316507.
Article29. Riffo-Vasquez Y, Spina D, Page C, Tormay P, Singh M, Henderson B, Coates A. Effect of Mycobacterium tuberculosis chaperonins on bronchial eosinophilia and hyper-responsiveness in a murine model of allergic inflammation. Clin Exp Allergy. 2004; 34:712–719. PMID: 15144461.
Article30. Ito T, Hasegawa A, Hosokawa H, Yamashita M, Motohashi S, Naka T, Okamoto Y, Fujita Y, Ishii Y, Taniguchi M, Yano I, Nakayama T. Human Th1 differentiation induced by lipoarabinomannan/lipomannan from Mycobacterium bovis BCG Tokyo-172. Int Immunol. 2008; 20:849–860. PMID: 18469327.
Article31. Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, García VE, Krutzik SR, Weis SE, Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008; 38:459–469. PMID: 18203140.32. Major T, Wohlleben G, Reibetanz B, Erb KJ. Application of heat killed Mycobacterium bovis-BCG into the lung inhibits the development of allergen-induced Th2 responses. Vaccine. 2002; 20:1532–1540. PMID: 11858859.
Article33. Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999; 163:3920–3927. PMID: 10490993.34. Lozes E, Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Vandenbussche P, Van Vooren JP, Drowart A, Ulmer JB, Liu MA. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997; 15:830–833. PMID: 9234526.
Article35. Wu J, Xu J, Cai C, Gao X, Li L, Zhong N. Ag85B DNA vaccine suppresses airway inflammation in a murine model of asthma. Respir Res. 2009; 10:51. PMID: 19531238.
Article36. Mori H, Yamanaka K, Matsuo K, Kurokawa I, Yasutomi Y, Mizutani H. Administration of Ag85B showed therapeutic effects to Th2-type cytokine-mediated acute phase atopic dermatitis by inducing regulatory T cells. Arch Dermatol Res. 2009; 301:151–157. PMID: 18633632.
Article37. von Mutius E, Pearce N, Beasley R, Cheng S, von Ehrenstein O, Björkstén B, Weiland S. International patterns of tuberculosis and the prevalence of symptoms of asthma, rhinitis, and eczema. Thorax. 2000; 55:449–453. PMID: 10817790.
Article38. Grüber C, Meinlschmidt G, Bergmann R, Wahn U, Stark K. Is early BCG vaccination associated with less atopic disease? An epidemiological study in German preschool children with different ethnic backgrounds. Pediatr Allergy Immunol. 2002; 13:177–181. PMID: 12144639.
Article39. Chernousova LN, Smirnova TG, Afanasieva EG, Karpov VL, Timofeev AV. Ex vivo production of interferon-gamma, tumor necrosis factor-alpha, and interleukin-6 by mouse macrophages during infection with M. bovis and M. tuberculosis H37Rv. Bull Exp Biol Med. 2007; 144:709–712. PMID: 18683503.40. Yoshida M, Leigh R, Matsumoto K, Wattie J, Ellis R, O'Byrne PM, Inman MD. Effect of interferon-γ on allergic airway responses in interferon-γ-deficient mice. Am J Respir Crit Care Med. 2002; 166:451–456. PMID: 12186819.41. van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, Welliver RC. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol. 2000; 62:257–266. PMID: 11002257.
Article42. Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002; 88:584–591. PMID: 12086366.
Article43. McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008; 181:4089–4097. PMID: 18768865.
Article44. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135. PMID: 17083726.
Article45. Barlan I, Bahceciler NN, Akdis M, Akdis CA. Bacillus Calmette-Guerin, Mycobacterium bovis, as an immunomodulator in atopic diseases. Immunol Allergy Clin North Am. 2006; 26:365–377. PMID: 16701150.
Article46. Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V, Bihl F, Erard F, Botha T, Drennan M, Soler MN, Le Bert M, Schnyder B, Ryffel B. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004; 6:946–959. PMID: 15310472.
Article47. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007; 9:1087–1098. PMID: 17359235.
Article48. Simons MP, Moore JM, Kemp TJ, Griffith TS. Identification of the mycobacterial subcomponents involved in the release of tumor necrosis factor-related apoptosis-inducing ligand from human neutrophils. Infect Immun. 2007; 75:1265–1271. PMID: 17194806.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of carbohydrate moieties of sparganum proteins of the snake, mouse and those of adult worm
- Attenuation of airway inflammation by simvastatin and the implications for asthma treatment: is the jury still out?
- Dose dependence and durability of the therapeutic effects of Asparagus cochinchinensis fermented extract in an ovalbumin-challenged asthma model
- Regulation of Mucin Exocytosis in Airway Secretory Cells
- Development of the mucociliary system in the eustachian tube and middle ear: murine model