Korean J Hematol.
2005 Jun;40(2):65-74. 10.5045/kjh.2005.40.2.65.
Eugenol Induces a Reactive Oxygen Species-mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells
- Affiliations
-
- 1Department of Internal Medicine, Holy Family Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea. blood@medimail.co.kr
- 2College of Pharmacy, Kyung Hee University, Seoul, Korea.
- KMID: 2252340
- DOI: http://doi.org/10.5045/kjh.2005.40.2.65
Abstract
- BACKGROUND
Eugenol is a major component of the essential oil isolated from Eugenia caryophyllata (Myrtaceae), and has been widely used as a traditional medicine. In this study, the effects of eugenol on the cytotoxicity, induction of apoptosis and putative pathways of its actions were investigated in human promyelocytic leukemia cells (HL-60).
METHODS
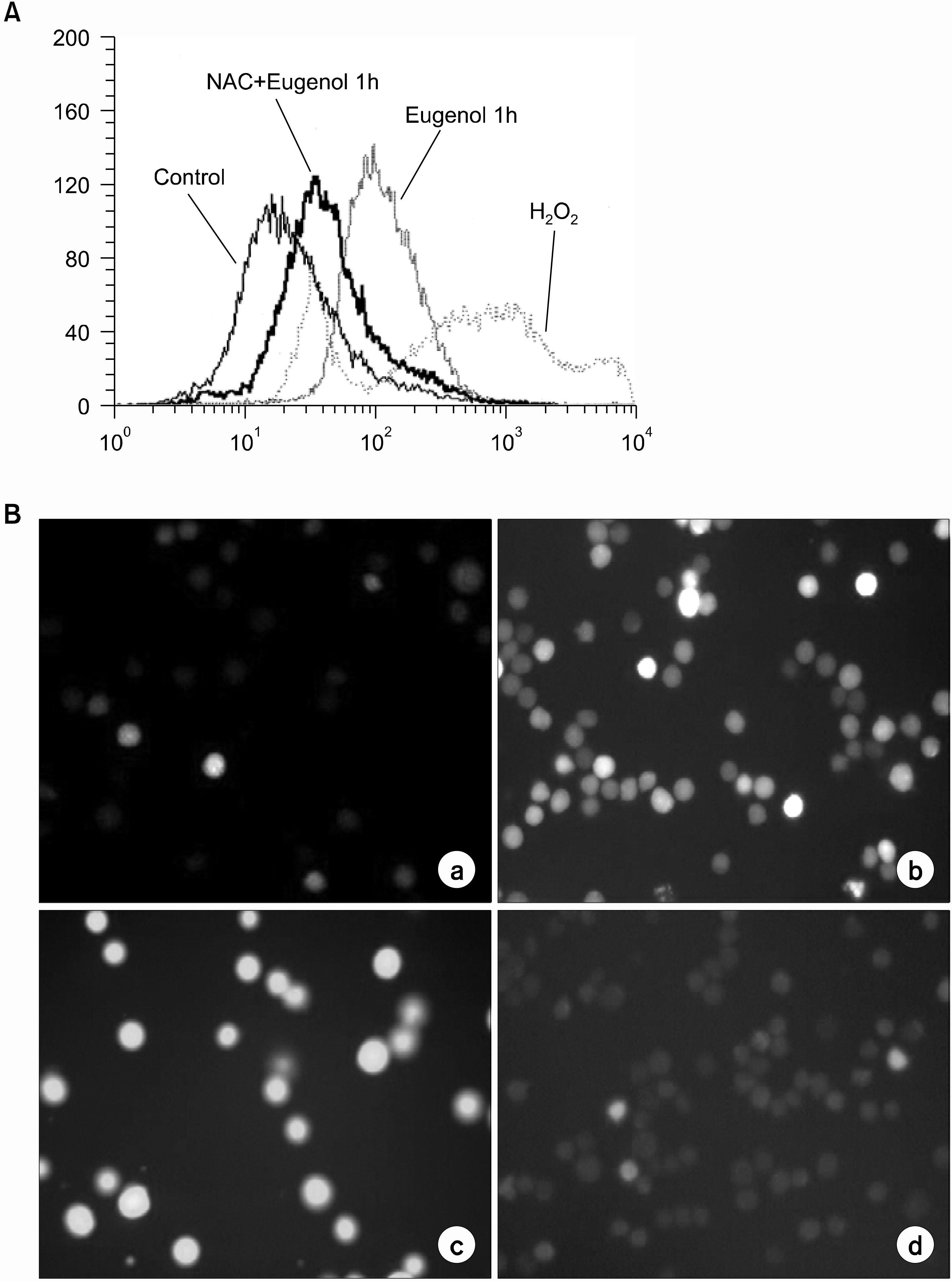
After applying eugenol to cultured HL-60, the changes in the mitochondrial membrane potential of the cells were monitored after double staining with propidium iodide and rhodamine 123, with 2', 7'-dicholorofluorescin diacetate was used to measure of levels of reactive oxygen species (ROS)
RESULTS
Eugenol was shown to be a potent inducer of apoptosis; transducing the apoptotic signal via ROS generation; thereby, inducing mitochondrial permeability transition (MPT) and cytochrome c release to the cytosol. The production of ROS, mitochondrial alteration and subsequent apoptotic cell death in eugenol-treated cells were blocked by the antioxidant, N-acetylcystein (NAC).
CONCLUSION
Taken together, the present study has demonstrated that eugenol induces ROS-mediated mitochondrial permeability transition and resultant cytochrome c release.
Keyword
MeSH Terms
Figure
Reference
-
1). Harborne JB, Baxter H. Phytochemical dictionary. Washington DC: Taylor and Francis;1993. p. 1765.2). Zheng GQ, Kenney PM, Lam LK. Sesquiterpenes from clove (Eugenia caryophyllata). J Nat Prod. 1992; 55:999–1003.3). Miyazawa M, Hisama M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhymurium TA1535/pSK1002 umu test. J Agric Food Chem. 2001; 49:4019–25.4). Ogata M, Hoshi M, Urano S, Endo T. Antioxidant activity of eugenol and related monomeric and dimeric compounds. Chem Pharm Bull. 2000; 48:1467–9.
Article5). Park IK, Lee HS, Lee SG, Park JD, Ahn YJ. Insecticidal and fumigant activities of Cinnamomumca-ssia bark-derived materials against Mechoris ursulus (Coleoptera: Attelabidae). J Agric Food Chem. 2000; 48:2528–31.6). Atsumi T, Iwakura I, Fujisawa S, Ueha T. Reactive oxygen species generation and photo-cytotoxicity of eugenol in solutions of various pH. Biomaterials. 2001; 22:1459–66.
Article7). Chogo JB, Crank G. Chemical composition and biological activity of the Tanzanian Plant Ocimum-suave. J Nat Prod. 1981; 42:308–11.8). Asha MK, Prashanth D, Murali B, Padmaja R, Amit A. Anthelmintic activity of essential oil of Ocimum sanctum and eugenol. Fitoterapia. 2001; 72:669–70.
Article9). Wright SE, Baron DA, Heffner JE. Intravenous eugenol causes hemorrhagic lung edema in rats: proposed oxidant mechanisms. J Lab Clin Med. 1995; 125:257–64.10). Suzuki Y, Sugiyama K, Furata H. Eugenol-mediated superoxide generation and cytotoxicity in guinea pig neutrophils. Jpn J Pharmacol. 1985; 39:381–6.
Article11). McDonald JW, Hoffner JE. Eugenol causes oxidant-mediated edema in isolated perfused rabbit lungs. Am Rev Respir Dis. 1991; 143:806–9.
Article12). Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995; 7:337–43.
Article13). Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995; 130:157–67.
Article14). Kaufmann SH. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989; 49:5870–8.15). Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996; 2:574–7.
Article16). Bhalla K, Ibrado AM, Tourkina E, et al. High-dose mitoxantrone induces programmed cell death or apoptosis in human myeloid leukemia cells. Blood. 1993; 15:3133–40.
Article17). Plumb JA, Milroy R, Kaye SB. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989; 49:4435–40.18). Lee KT, Kim JI, Rho YS, et al. Hypericin induces both differentiation and apoptosis in human promyelocytic leukemia HL-60 cells. Biol Pharm Bull. 1999; 22:1271–4.
Article19). Watabe M, Kawazoe N, Masuda Y, Nakajo S, Nakaya K. Bcl-2 protein inhibits bufalin-induced apoptosis through inhibition of mitogen-activated protein kinase activation in human leukemia U937 cells. Cancer Res. 1997; 57:3097–100.20). Neumann C, Boubakari , Grunert R, Bednarski PJ. Nicotinamide adenine dinucleotide phosphate-regenerating system coupled to a glutahione-reductase microtitier method for determination of total glutathione concentration in adherent growing cancer cell lines. Anal Biochem. 2003; 320:170–8.21). Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/cas pase-9 complex initiates an apoptotic protease cascade. Cell. 1997; 14:479–89.22). Bradham CA, Qian T, Streetz K, Trautwein C, Brenner DA, Lemasters JJ. The mitochondrial permeability transition is required fortumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998; 18:6353–64.23). Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000; 5:415–8.24). Catisti R, Vercesi AE. The participation of pyridine nucleotides redox state and reactive oxygen in the fatty acid-induced permeability transition in rat liver mitochondria. FEBS Lett. 1999; 464:97–101.
Article25). Simizu S, Takada M, Umezawa K. Imoto M. Requirement of caspase-3 (-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998; 273:26900–7.26). Duranteau J, Chandel NS, Kulisz A, Shao Z, Schu-macker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998; 273:11619–24.
Article27). Ghibelli L, Fanelli C, Rotilio G, et al. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998; 12:479–86.
Article28). Macho A, Hirsch T, Marzo I, et al. Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J Immunol. 1997; 158:4612–9.29). Beaver JP, Waring P. A decrease in intracellular glutathione concentration precedes the onset of apoptosis in murine thymocytes. Eur J Cell Biol. 1995; 68:47–54.30). van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cot-greave IA, Orrenius S, Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem. 1996; 271:15420–7.
Article31). Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999; 93:268–77.
Article32). Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997; 278:1073–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor activity and mitochondrial stability of disulfiram in HL-60 cells
- Selective Effects of Curcumin on CdSe/ZnS Quantum-dot-induced Phototoxicity Using UVA Irradiation in Normal Human Lymphocytes and Leukemia Cells
- Activation of Caspase-3 in Hydrogen Peroxide-Induced Apoptosis of Human Leukemia HL 60 Cells
- Hydroxydibenzoylmethane induces apoptosis through repressing ornithine decarboxylase in human promyelocytic leukemia HL-60 cells
- Arsenic Trioxide Induces Apoptosis of HL-60 Cells via Activation of Intrinsic Caspase Protease with Mitochondrial Dysfunction