J Korean Soc Transplant.
2012 Dec;26(4):248-253. 10.4285/jkstn.2012.26.4.248.
Changes in Serum Cytokine Profile after AEB071 (Sotrastaurin) or Tacrolimus versus Their Combinations in Rat Heterotopic Cardiac Allografts
- Affiliations
-
- 1The Research Institute for Transplantation, Yonsei University College of Medicine, Seoul, Korea. yukim@yuhs.ac
- 2Department of Transplantation Surgery, Yonsei University Health System, Seoul, Korea.
- 3Graduate Program of Nanoscience and Technology, Yonsei University, Seoul, Korea.
- 4BK21 for Medical Science, Yonsei University Health System, Seoul, Korea.
- KMID: 2202427
- DOI: http://doi.org/10.4285/jkstn.2012.26.4.248
Abstract
- BACKGROUND
AEB071, an orally available PKC inhibitor, prevents organ rejection after transplantation in rodents and man. Furthermore, pro-inflammatory cytokines and inflammatory processes are important mediators of transplanted organ rejection. We therefore examined whether single or combination therapies of AEB071 and/or tacrolimus affect cytokine profiles in a rat cardiac allograft model.
METHODS
AEB071 (60 mg/kg twice a day) and tacrolimus (0.6 or 1.2 mg/kg once a day) were orally administered daily after cardiac transplantation. Interferon (IFN)-gamma, interleukin (IL)-1beta, IL-2, IL-4, IL-6, IL-10, and tumor necrosis factor (TNF)-alpha levels in serum were subsequently measured 5 days after cardiac transplantation using a multiplex protein assay system.
RESULTS
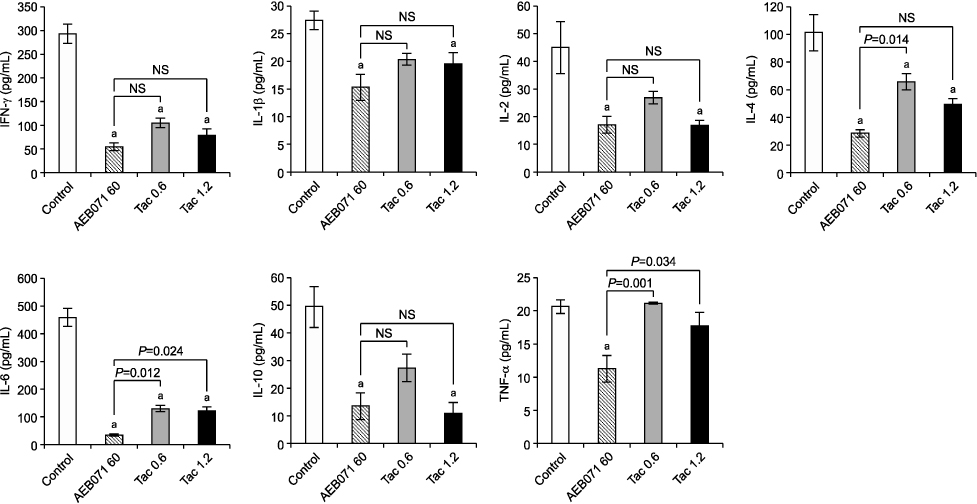
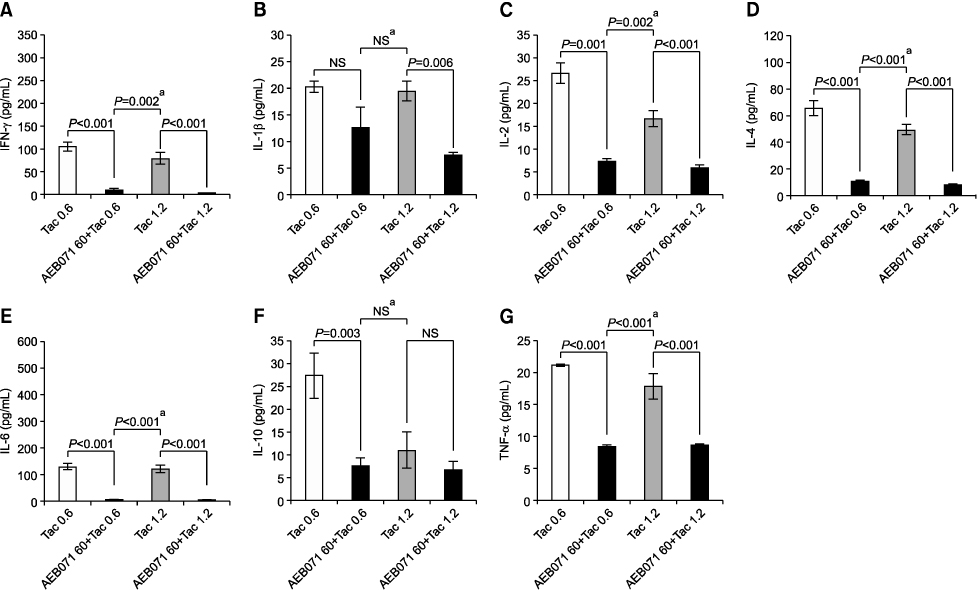
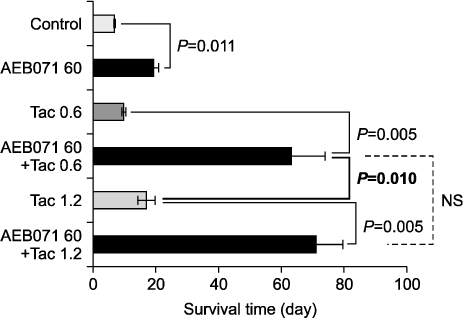
All cytokine levels were significantly depressed in cardiac transplanted rats treated with AEB071, whereas tacrolimus only reduced IFN-gamma, IL-2, IL-4, IL-6, and IL-10 levels. When administered in combination, AEB071 and low- or high-dose tacrolimus had additive effects on IFN-gamma, IL-4, IL-6, and TNF-alpha.
CONCLUSIONS
These results suggest that AEB071 inhibits T cell activation by blocking the production of proinflammatory cytokines, and that tacrolimus combined with AEB071 can effectively regulate inflammatory cytokines in the transplantation setting.
MeSH Terms
-
Animals
Cytokines
Heart Transplantation
Immunosuppression
Interferons
Interleukin-10
Interleukin-2
Interleukin-4
Interleukin-6
Interleukins
Pyrroles
Quinazolines
Rats
Rejection (Psychology)
Rodentia
Tacrolimus
Transplantation, Homologous
Transplants
Tumor Necrosis Factor-alpha
Cytokines
Interferons
Interleukin-10
Interleukin-2
Interleukin-4
Interleukin-6
Interleukins
Pyrroles
Quinazolines
Tacrolimus
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Manicassamy S. Sotrastaurin, a protein kinase C inhibitor for the prevention of transplant rejection and treatment of psoriasis. Curr Opin Investig Drugs. 2009. 10:1225–1235.2. Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003. 376(Pt 3):545–552.
Article3. Merani S, Pawlick RL, Edgar RL, Toso C, Emamaullee J, Anderson CC, et al. Protein kinase C inhibitor, AEB-071, acts complementarily with cyclosporine to prevent islet rejection in rats. Transplantation. 2009. 87:59–65.
Article4. Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997. 9:161–167.
Article5. Baier G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev. 2003. 192:64–79.
Article6. Pfeifhofer C, Gruber T, Letschka T, Thuille N, Lutz-Nicoladoni C, Hermann-Kleiter N, et al. Defective IgG2a/2b class switching in PKC alpha-/- mice. J Immunol. 2006. 176:6004–6011.7. Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, et al. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996. 273:788–791.8. Tan J, Maass DL, White DJ, Horton JW. Effects of burn injury on myocardial signaling and cytokine secretion: possible role of PKC. Am J Physiol Regul Integr Comp Physiol. 2007. 292:R887–R896.
Article9. Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997. 385:83–86.
Article10. Marsland BJ, Kopf M. T-cell fate and function: PKC-theta and beyond. Trends Immunol. 2008. 29:179–185.11. Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, et al. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J Pharmacol Exp Ther. 2009. 330:792–801.
Article12. Fang YH, Joo DJ, Lim BJ, Kim JY, Kim MS, Jeong HJ, et al. AEB-071 versus tacrolimus monotherapy to prevent acute cardiac allograft rejection in the rat: a preliminary report. Transplant Proc. 2010. 42:976–979.
Article13. Fang YH, Joo DJ, Lim BJ, Huh KH, Kim MS, Suh H, et al. The effects of AEB071 (sotrastaurin) with tacrolimus on rat heterotopic cardiac allograft rejection and survival. J Surg Res. 2011. 171:e133–e137.
Article14. Wagner J, von Matt P, Sedrani R, Albert R, Cooke N, Ehrhardt C, et al. Discovery of 3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]pyrrole-2,5-dione (AEB071), a potent and selective inhibitor of protein kinase C isotypes. J Med Chem. 2009. 52:6193–6196.
Article15. Dugré FJ, Gaudreau S, Belles-Isles M, Houde I, Roy R. Cytokine and cytotoxic molecule gene expression determined in peripheral blood mononuclear cells in the diagnosis of acute renal rejection. Transplantation. 2000. 70:1074–1080.
Article16. Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994. 76:241–251.
Article17. Strom TB, Roy-Chaudhury P, Manfro R, Zheng XX, Nickerson PW, Wood K, et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996. 8:688–693.
Article18. Karczewski J, Karczewski M, Glyda M, Wiktorowicz K. Role of TH1/TH2 cytokines in kidney allograft rejection. Transplant Proc. 2008. 40:3390–3392.
Article19. Leanderson T, Lundgren E, Ruuth E, Borg H, Persson H, Coutinho A. B-cell growth factor: distinction from T-cell growth factor and B-cell maturation factor. Proc Natl Acad Sci U S A. 1982. 79:7455–7459.
Article20. Zavorotinskaya T, Tomkinson A, Murphy JE. Treatment of experimental asthma by long-term gene therapy directed against IL-4 and IL-13. Mol Ther. 2003. 7:155–162.
Article21. Grewal IS. Overview of TNF superfamily: a chest full of potential therapeutic targets. Adv Exp Med Biol. 2009. 647:1–7.
Article22. Sabiston DC, Townsend CM. Sabiston textbook of surgery: the biological basis of modern surgical practice. 2008. 18th ed. Philadelphia, USA: Saunders/Elsevier.23. Sonkar GK, Singh RG. Usha. Evaluation of serum tumor necrosis factor alpha and its correlation with histology in chronic kidney disease, stable renal transplant and rejection cases. Saudi J Kidney Dis Transpl. 2009. 20:1000–1004.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four Cases of Atopic Dermatitis with Topical Tacrolimus Therapy
- Effect of tacrolimus XL on variance coefficients in comparison with twice daily tacrolimus, and relationship with serum creatinine concentrations in kidney transplant recipients
- Risk of graft loss on once-daily versus twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- Chronic urticaria treated with tacrolimus
- Two Cases of Lichen Nitidus Treated with Topical 0.1% Tacrolimus