J Korean Neurosurg Soc.
2013 Jun;53(6):337-341. 10.3340/jkns.2013.53.6.337.
Epigenetic Regulation in the Brain after Spinal Cord Injury : A Comparative Study
- Affiliations
-
- 1Department of Rehabilitation Medicine, Wonju Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea. kimrehab@yonsei.ac.kr
- 2Department of Rehabilitation Medicine and Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Anatomy, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Neurosurgery, College of Medicine, Chosun University, Gwangju, Korea.
- KMID: 2190820
- DOI: http://doi.org/10.3340/jkns.2013.53.6.337
Abstract
OBJECTIVE
After spinal cord injury (SCI), functional and structural reorganization occurs at multiple levels of brain including motor cortex. However, the underlying mechanism still remains unclear. The current study was performed to investigate the alterations in the expression of the main regulators of neuronal development, survival and death, in the brain following thoracic contusive SCI in a mouse model.
METHODS
Eight-week-old female imprinting control region mice (n=60; 30-35 g) were used in this study. We analyzed the expression levels of regulators such as brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), nerve growth factor (NGF) and histone deacetylase (HDAC) 1 in the brain following thoracic contusive SCI.
RESULTS
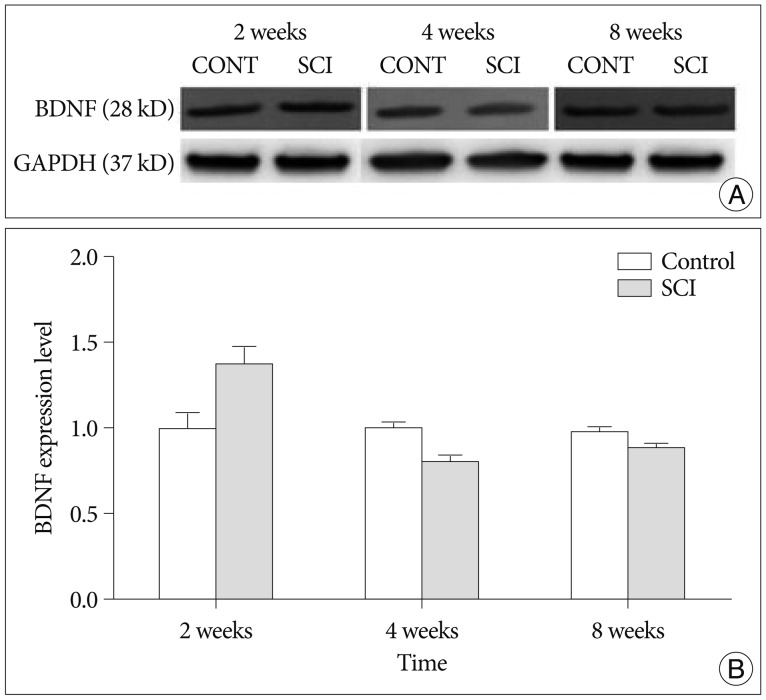
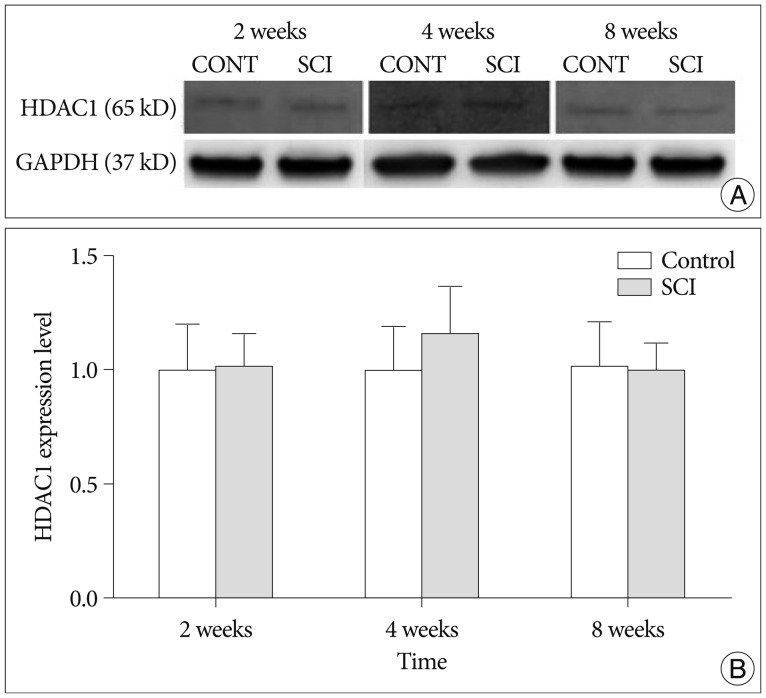
The expression of BDNF levels were elevated significantly compared with control group at 2 weeks after injury (p<0.05). The expression of NGF levels were elevated at 2, 4 weeks compared with control group, but these difference were not significant (p>0.05). The GDNF levels were elevated at 2 week compared with control group, but these differences were not significant (p>0.05). The difference of HDAC1 levels were not significant at 2, 4 and 8 weeks compared with control group (p>0.05).
CONCLUSION
These results demonstrate that the upregulation of BDNF may play on important role in brain reorganization after SCI.
MeSH Terms
-
Animals
Brain
Brain-Derived Neurotrophic Factor
Epigenomics
Female
Glial Cell Line-Derived Neurotrophic Factor
Histone Deacetylases
Humans
Mice
Motor Cortex
Nerve Growth Factor
Neurons
Spinal Cord
Spinal Cord Injuries
Up-Regulation
Brain-Derived Neurotrophic Factor
Glial Cell Line-Derived Neurotrophic Factor
Histone Deacetylases
Nerve Growth Factor
Figure
Reference
-
1. Boutillier AL, Trinh E, Loeffler JP. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J Neurochem. 2003; 84:814–828. PMID: 12562525.
Article2. Bregman BS, Goldberger ME. Infant lesion effect : II. Sparing and recovery of function after spinal cord damage in newborn and adult cats. Brain Res. 1983; 285:119–135. PMID: 6616260.3. Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998; 10:3918–3922. PMID: 9875370.
Article4. Burns SP, Golding DG, Rolle WA Jr, Graziani V, Ditunno JF Jr. Recovery of ambulation in motor-incomplete tetraplegia. Arch Phys Med Rehabil. 1997; 78:1169–1172. PMID: 9365343.
Article5. Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, et al. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 2004; 127(Pt 1):34–44. PMID: 14534158.
Article6. Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988; 332:444–445. PMID: 3352741.
Article7. Cohen LG, Roth BJ, Wassermann EM, Topka H, Fuhr P, Schultz J, et al. Magnetic stimulation of the human cerebral cortex, an indicator of reorganization in motor pathways in certain pathological conditions. J Clin Neurophysiol. 1991; 8:56–65. PMID: 2019651.
Article8. Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002; 19:43–51. PMID: 11852977.
Article9. Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury : an electroencephalographic study. Neurology. 1998; 50:1115–1121. PMID: 9566404.
Article10. Hajebrahimi Z, Mowla SJ, Movahedin M, Tavallaei M. Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci Lett. 2008; 441:261–266. PMID: 18585435.
Article11. Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997; 386:495–498. PMID: 9087408.
Article12. Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006; 198:401–415. PMID: 16443221.
Article13. Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001; 294:1945–1948. PMID: 11729324.
Article14. Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003; 4:944–947. PMID: 14528264.
Article15. McKinley PA, Jenkins WM, Smith JL, Merzenich MM. Age-dependent capacity for somatosensory cortex reorganization in chronic spinal cats. Brain Res. 1987; 428:136–139. PMID: 3815108.
Article16. Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, et al. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999; 19:2069–2080. PMID: 10066260.
Article17. Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996; 16:785–807. PMID: 8551360.
Article18. Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991; 252:1857–1860. PMID: 1843843.
Article19. Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001; 2:263–273. PMID: 11283749.
Article20. Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996; 379:247–250. PMID: 8603699.
Article21. Seidah NG, Chrétien M. Proprotein and prohormone convertases : a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999; 848:45–62. PMID: 10701998.
Article22. Waller A. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibers. Phil Trans R Soc Lond. 1850; 140:423.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Failure of initial neurologic assessment for SCIWORA with brain injury in 22-month-old patient
- Delayed Spinal Cord Injury Following Low Voltage Electrical Accident
- Spinal Cord Injury Followign Electrical Accidents : Case Reports
- Paraplegia Following Spinal Cord Contusion from an Indirect Gunshot Injury
- The Role of GABA in Spinal Cord Injury