J Korean Acad Conserv Dent.
2002 Jan;27(1):1-11. 10.5395/JKACD.2002.27.1.001.
The levels of interleukin-2, interferon-gamma, interleukin-4 and T lymphocyte subpopulations in rat pulpal inflammation induced experimentally by specific bacteria
- Affiliations
-
- 1Department of Conservative Dentistry, Graduate School, Seoul National University, Korea.
- KMID: 2175542
- DOI: http://doi.org/10.5395/JKACD.2002.27.1.001
Abstract
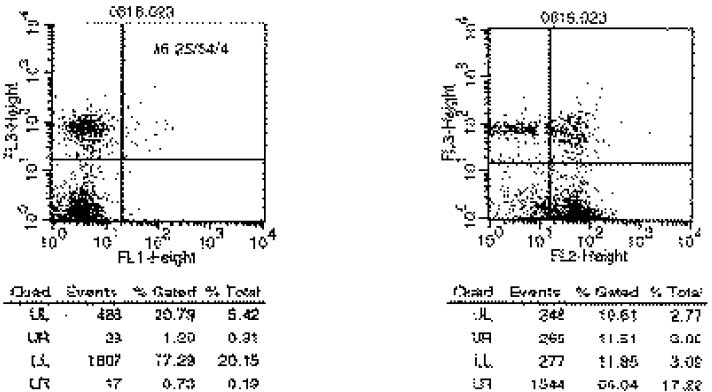
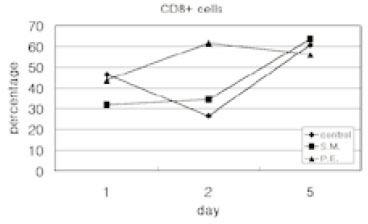
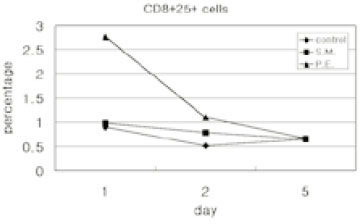
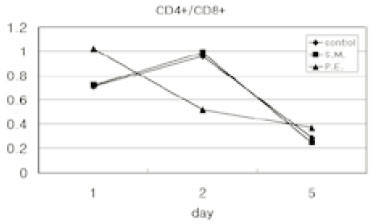
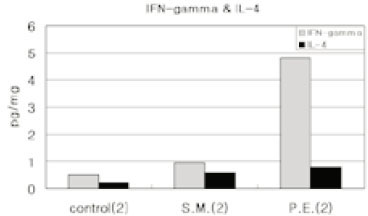
- Immune responses associated with bacterial infection involve various inflammatory cells. Clinical symptoms and pathologic features are particularly influenced by the predominant cells. Among inflammatory cells, T cells have the heterogenity. T cells may develop into the mature cells expressing the cell surface markers with different functions and T helper cells are categorized into Th1 and Th2 cells based on their different patterns of cytokine production. The objective of this study was to investigate the change of expression of surface markers on T cells and the Th1/Th2 immune response in pulpal inflammation associated with specific bacteria. We experimentally induced pulpal inflammation in rat incisors by drilling without coolant and innoculated with Streptococcus mutans (S.M. group), Porphyromonas endodontalis (P.E. group), or only sterile cotton (control group). After 1, 2, and 5 days, mandibular incisors were extracted and the pulp tissues were extirpated. The expressions of IL-2 recepters (CD25) and ICAM-1 (CD54) on CD4+ and CD8+ cells in the pulps were determined using a flow cytometer, and the concentration of IL-2, IFN-gamma and IL-4 was measured by enzyme-linked immunosorbent assay. The results were as follows; 1. In the S.M. group, CD4+ cells were more increased at 2nd day than 1st day and in the P.E. group, CD8+ cells were more increased at 2nd day than 1st day. 2. The percentages of CD4+, CD4+25+ and CD4+54+ cells were decreased in the pulp tissues at 5th day after irritation in all groups. 3. The ratios of CD4+/CD8+, CD4+/CD4+25+ and CD4+/CD4+54+ in the pulps at 2nd day after irritation by P. endodontalis were significantly lower than the other groups. 4. The higher concentrations of IFN-gamma than IL-4 in the pulps at 2nd day after irritation by P. endodontalis showed that T helper 1 reaction were predominant in the early stage of the pulpal inflammation induced by P. endodontalis. 5. The higher concentrations of IL-4 than IFN-gamma in the pulps at 1st day and 5th day after irritation by S. mutans were measured but the differences were not significant.
MeSH Terms
-
Animals
Bacteria
Bacterial Infections
Incisor
Inflammation
Intercellular Adhesion Molecule-1
Interferon-gamma
Interleukin-2
Interleukin-4
Lymphocyte Subsets
Lymphocytes
Mandrillus
Porphyromonas endodontalis
Rats
Streptococcus mutans
T-Lymphocytes
T-Lymphocytes, Helper-Inducer
Th2 Cells
Intercellular Adhesion Molecule-1
Interferon-gamma
Interleukin-2
Interleukin-4
Figure
Reference
-
1. Marton IJ, Kiss C. Characterization of inflammatory cell infiltrate in dental periapical lesions. Int Endod J. 1993. 26:131–136.
Article2. Fouad AF. IL-1alpha and TNF-alpha expression in early periapical lesions of normal and immunodeficient mice. J Dent Res. 1997. 76(9):1548–1554.
Article3. Back SH, Lim SS. An immunohistochemical study on the immunoglobulin g subclasses of the experimentally induced rat pulp and periapical pathoses. J Korean Acad Conserv Dent. 1991. 16:41–59.4. Kopp W, Schwarting R. Differentiation of T lymphocyte subpopulations, macrophages, and HDA-DR-restricted cells of apical granulatuib tissue. J Endod. 1989. 15:72–75.
Article5. Stashenko P, Yu SM, Wang CY. Kinetics of immune cell and bone resorptive responses to endodontic infections. J Endod. 1992. 18:422–426.
Article6. Kawashima N, Okiji T, Kosaka T. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars; a quantitative immunohistochemcial study. J Endod. 1996. 22:311–316.
Article7. Matsuo T, Ebisu S, Shimabukuro Y. Quantitative analysis of immunocompetent cells in human periapical lesions; Correlations with clinical findings of the involved teeth. J Endod. 1992. 8:497–500.
Article8. Oh TS, Lim SS. Flow cytometric analysis of lymphocyte and cycling cell distribution in periapical lesions. J Korean Acad Conserv Dent. 1993. 18:317–340.9. Sol MA, Tkaczuk J. Voigt JJ Characterization of lymphocyte subpopulations in periapical lesions by flow cytometry. Oral Microbiol Immunol. 1998. 13:253–258.
Article10. Abbs AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996. 383:787–793.
Article11. Yamasaki M, Nakane A, Kumazawa M. Endotoxin and Gram-negative bacteria in the rat periapical lesions. J Endod. 1992. 18(10):501–504.
Article12. Gemmell E, Winning TA, Bird PS. Cytokine profiles of lesional and splrenic T cells in Porphyromonas gingivalis infection in a murine model. J Periodontol. 1998. 69:1131–1138.
Article13. Byun HY, Lim SS, Park DS. Levels of tnf-α,-β, il-1β, tgf-β1 and their relationship with the presence of specific black pigmented bacteria in periapical and pulpal diseases. J Korean Acad Conserv Dent. 1999. 24:1–12.14. Hren NI, Gubina M, Ihan A. Cytotoxic T lymphocytes versus streptococcal colonization in periapical granulomas. J Endod. 1999. 25:239–242.
Article15. Speer ML, Madonia JV, Heuer MA. Quantitative evaluation of the immunocompetence of the dental pulp. J Endod. 1977. 3:418–423.
Article16. Jontell M, Gunraj N, Bergenholtz G. Immunocompetent cells in normal dental pulp. J Dent Res. 1986. 66:1149–1153.17. Pulver WH, Taubman MA, Smith OJ. Immune components in normal and inflamed human dental pulp. Arch Oral Biol. 1977. 22:103–111.
Article18. Lee WC, Lim SS. Lymphocytes population in relation to clinical symptoms in irreversible pulpitis. J Korean Acad Conserv Dent. 1995. 20:235–249.19. Hahn CL, Falkler WA, Siegel MA. A study of T and B cells in pulpal pathosis. J Endod. 1989. 15:20–26.
Article20. Mangkornkarn C, Steiner JC, Bohman R, Lindemann RA. Flow cytometric analysis of human dental pulp. J Endod. 1991. 17:49–53.21. Kotylo HA, Sample RB, Redmond NL. Reference ranges for lymphocyte subsets. A comparison of standard vs rapid whole-blood lysis techniques. Arch Pathol Lab Med. 1991. 115:181–184.22. Kim SA, Bae KS, Lim SS. Flow cytometric analysis of lymphocytes in normal and inflamed pulp. J Korean Acad Conserv Dent. 1997. 22:374–387.23. Okiji T, Morita I, Sunada I, Murota S. Involvement of arachidonic acid metabolites in increases in vascular permeability in experimental dental pulpal inflammation in the rat. Arch Oral Biol. 1989. 34:523–528.
Article24. Bergenholtz G, Nagaoka S, Jontell M. Class II antigen expressing cells in experimentally induced pulpitis. Int Endod J. 1991. 24:8–14.
Article25. Okiji T, Morita I, Kobayashi C, Sunada I, Murota S. Arachidonic-acid metabolosm in normal and experimentally-inflamed rat dental pulp. Arch Oral Biol. 1987. 32(10):723–727.
Article26. Hashimoto S, Maeda M, Yamakita J, Nakamura Y. Effects of zinc oxide-eugenol on leucocyte number and lipoxygenase products in artificially inflamed rat dental pulp. Arch Oral Biol. 1990. 35:87–93.
Article27. Suzuki N, Okiji T, Suda H. Enhanced expansion of activation - association molecules on macrophages of heterogeneous populations in expanding periapical lesions in rat molars. Arch Oral Biol. 1999. 44:67–79.
Article28. Rauschenberger CR, Bailey JC, Cootauco CJ. Detection of human IL-2 in normal and inflamed dental pulps. J Endod. 1997. 23:366–370.
Article29. Nguyen T, Wang R, Russeu JH. IL-12 enhances IL-2 function by inducing CD2 expression through a p38 mitogen-activated protein kinases pathway. Eur J Immunol. 2000. 30:1445–1452.
Article30. Okiji T, Morita I, Sunada I, Murota S. The role of Leukotriene B4 in neutrophil infiltration in experimentally-induced inflammation of rat tooth pulp. J Dent Res. 1991. 70(1):34–37.
Article31. Wedenberg C, Borstein R. Pulpal reactions in rat incisors to Caridex. Aust Dent J. 1990. 35(6):505–508.
Article32. Kettering JD, Torbinejad M, Jones SL. Specificity of antibodies present in human periapical lesions. J Endod. 1991. 17:213–216.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interleukin-6 and interleukin-10 in experimentally induced rat pulpal inflammation
- The effects of interleukin-4 and interferon-gamma on the expression of FcepsilonR II on B cells
- The role of interleukin-6 and interleukin-10 in human pulpal inflammation
- Effects of interferon-alpha and -gamma on lymphokine-activated killer cell activity induced by interleukin-2
- Effect of Interleukin-10 on Lipopolysaccahride/Interferon-gamma- Induced Chemokine Mig Gene Expression