Chonnam Med J.
2009 Apr;45(1):1-8. 10.4068/cmj.2009.45.1.1.
Oxidative Stress and Molecular Reactions in Arteriogenic Erectile Dysfunction
- Affiliations
-
- 1Department of Urology and Pathology, VA Boston Healthcare System and Boston University School of Medicine, Massachusetts, USA. kazadzoi@bu.edu
- KMID: 2172277
- DOI: http://doi.org/10.4068/cmj.2009.45.1.1
Abstract
- Vascular disorders are the leading causes of organic erectile dysfunction (ED). Most cases of arteriogenic ED are associated with vascular risk factors such as hypercholesterolemia, atherosclerosis, diabetes mellitus, hypertension, and smoking. These conditions impair vascular structure directly or indirectly by facilitating oxidative damage due to accumulation of reactive oxygen and nitrogen species such as superoxide (O2-), hydrogen peroxide (H2O2), hydroxyl radicals and peroxynitrite (O=NOO-). Clinical studies have shown that the degree of arteriogenic ED cannot be predicted exclusively by the arterial integrity, suggesting that it involves local changes within penile smooth muscle cells, endothelium, nerves, and microvasculature. It is thought that arterial occlusion and subsequent lack of erectile tissue perfusion, nutrient deficiency, hypoxia, and accumulation of waste products constitute an environment hospitable to free radical formation and detrimental to nitric oxide (NO) bioavailability. Studies of animal and cell culture models have revealed that exposure to ischemia and hypoxia triggers a cascade of cellular and molecular changes in the erectile tissue with devastating impact on its structure and function. These alterations involve cytotoxic radicals, nitric oxide synthase (NOS), eicosanoids, neurotoxic elements, and growth factors and lead to impairment of NO/cGMP pathway, smooth muscle dysfunction, microvascular damage, and neurodegeneration. Ultimately, these events lead to erectile tissue fibrosis and the failure of penile veno-occlusive mechanism. These local changes in the hypoxic erectile tissue may explain the failure of revascularization surgery to completely cure arteriogenic ED and imply the need for newer therapeutic strategies to remove cytotoxic and neurotoxic elements from the chronically ischemic penis.
MeSH Terms
-
Animals
Anoxia
Atherosclerosis
Biological Availability
Cell Culture Techniques
Diabetes Mellitus
Eicosanoids
Endothelium
Erectile Dysfunction
Fibrosis
Hydrogen Peroxide
Hypercholesterolemia
Hypertension
Intercellular Signaling Peptides and Proteins
Ischemia
Male
Microvessels
Muscle, Smooth
Myocytes, Smooth Muscle
Nitric Oxide
Nitric Oxide Synthase
Nitrogen
Oxidative Stress
Oxygen
Perfusion
Peroxynitrous Acid
Risk Factors
Smoke
Smoking
Superoxides
Waste Products
Eicosanoids
Hydrogen Peroxide
Intercellular Signaling Peptides and Proteins
Nitric Oxide
Nitric Oxide Synthase
Nitrogen
Oxygen
Peroxynitrous Acid
Smoke
Superoxides
Waste Products
Figure
Reference
-
1. Krane RJ, Goldstein I, Saenz de Tejada I. Impotence. N Engl J Med. 1989. 32:1648–1659.
Article2. Grein U, Schubert GE. Arteriosclerosis of penile arteries: histological findings and their significance in the treatment of erectile dysfunction. Urol Int. 2002. 68:261–264.
Article3. Siroky MB, Azadzoi KM. Vasculogenic Erectile Dysfunction: Newer Therapeutic Strategies. J Urol. 2003. 170:S24–S29.
Article4. Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 203. 89:251–253.
Article5. Kloner RA, Mullin SH, Shook T, Matthews R, Mayeda G, Burstein S, et al. Erectile Dysfunction in the Cardiac Patient: How common and should we treat it? J Urol. 2003. 170:S46–S50.6. Levine FJ, Greenfield AJ, Goldstein I. Arteriographically-determined occlusive disease within the hypogastric-cavernous bed in impotent patients following blunt perineal and pelvic trauma. J Urol. 1990. 144:1147–1153.
Article7. Azadzoi KM, Park K, Andry C, Goldstein I, Siroky MB. Relationship between cavernosal ischemia and corporal veno-occlusive dysfunction in an animal model. J Urol. 1997. 157:1011–1017.
Article8. Aboseif SR, Breza J, Orvis BR, Lue TF, Tanagho EA. Erectile response to acute and chronic occlusion of the internal pudendal and penile arteries. J Urol. 1989. 141:398–402.
Article9. Munarriz RM, Yan QR, Nehra A, Udelson D, Goldstein I. Blunt trauma: the pathophysiology of hemodynamic injury leading to erectile dysfunction. J Urol. 1995. 153:1831–1840.
Article10. Azadzoi KM, Goldstein I, Siroky MB, Traish AM, Krane RJ, Saenz de Tejada I. Mechanisms of ischemia-induced cavernosal smooth muscle relaxation impairment in a rabbit model of vasculogenic erectile dysfunction. J Urol. 1998. 160:2216–2222.
Article11. Azadzoi KM, Krane RJ, Saenz de Tejada I, Goldstein I, Siroky MB. Relative roles of cyclooxygenase and nitric oxide synthase pathways in ischemia-induced increased contraction of cavernosal smooth muscle. J Urol. 1999. 161:1324–1328.
Article12. Azadzoi KM, Master TA, Siroky MB. Effect of chronic ischemia on constitutive and inducible nitric oxide synthase expression in erectile tissue. J Androl. 2004. 25:382–388.
Article13. Wespes E, Sattar AA, Golzarian J, Wery D, Daoud N, Schulman CC. Corporeal veno-occlusive dysfunction: predominantly intracavernous muscular pathology. J Urol. 1997. 157:1678–1680.
Article14. Nehra A, Goldstein I, Pabby A, Nugent M, Huang YH, de las Morenas A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996. 156:1320–1329.
Article15. Sattar AA, Salpigides G, Vanderhaeghen JJ, Schulman CC, Wespes E. Cavernous oxygen tension and smooth muscle fibers: relations and function. J Urol. 1995. 154:1736–1739.
Article16. Persson C, Diederichs W, Lue TF, Yen TS, Fishman IJ, McLin PH. Correlation of altered penile ultrastructure with clinical arterial evaluation. J Urol. 1989. 142:1462–1468.
Article17. Tarhan F, Kuyumcuoglu U, Kolsuz A, Ozgul A, Canguven O. Cavernous oxygen tension in the patients with erectile dysfunction. Int J Impot Res. 1997. 9:149–153.
Article18. Kim NN, Kim JJ, Hypolite J, Garcia-Diaz JF, Broderick GA, Tornheim K, et al. Altered contractility of rabbit penile corpus cavernosum smooth muscle by hypoxia. J Urol. 1996. 155:772–778.
Article19. Kim N, Vardi Y, Padma-Nathan H, Daley J, Goldstein I, Saenz de Tejada I. Oxygen tension regulates the nitric oxide pathway. Physiological role in penile erection. J Clin Invest. 1993. 91:437–442.
Article20. Daley JT, Brown ML, Watkins T, Traish AM, Huang YH, Moreland RB, et al. Prostanoid production in rabbit corpus cavernosum: I. regulation by oxygen tension. J Urol. 1996. 155:1482–1487.
Article21. Moreland RB. Is there a role of hypoxemia in penile fibrosis: a viewpoint presented to the Society for the Study of Impotence. Int J Impot Res. 1998. 10:113–120.
Article22. Darley-Usmar V, White R. Disruption of vascular signaling by the reaction of nitric oxide with superoxide: implication for cardiovascular disease. Exp Physiol. 1997. 82:305–316.
Article23. Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, et al. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide 1-42 and lipopolysaccharide-activated microglia. J Neurosci. 2002. 22:3484–3492.
Article24. Belló-Klein A, Bock PM, Travacio M, Senna SM, Llesuy S, de Bittencourt PI Jr, et al. Myocardial oxidative stress and antioxidants in hypertension as a result of nitric oxide synthase inhibition. Cardiovasc Toxicol. 2001. 1:43–50.
Article25. Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol. 2008. 28:5106–5119.
Article26. Kinlay S, Behrendt D, Fang JC, Delagrange D, Morrow J, Witztum JL, et al. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol. 2004. 43:629–634.
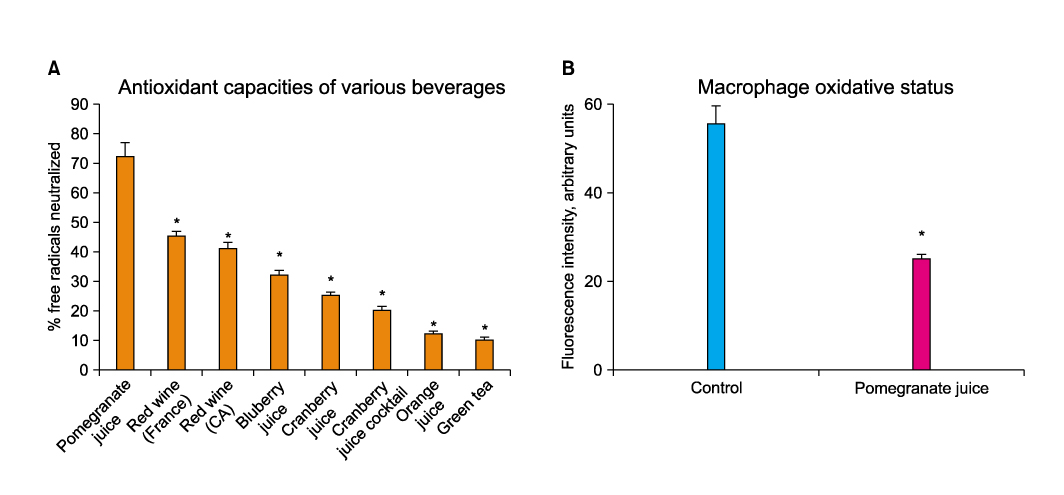
Article27. Fuhrman B, Lavy A, Aviram M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am J Clin Nutr. 1995. 61:549–554.
Article28. Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997. 17:2744–2752.
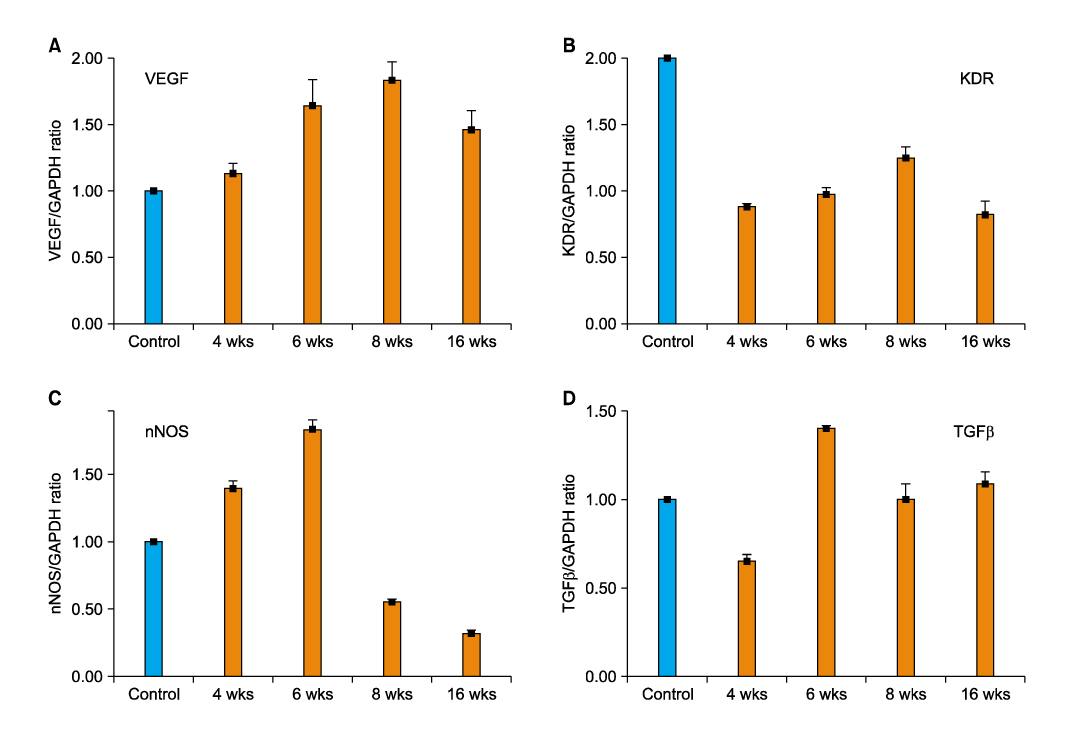
Article29. Wang T, Soker S, Atala A, Siroky MB, Azadzoi KM. Alterations in angiogenic growth factors and neuronal nitric oxide synthase expression in chronic cavernosal ischemia. Int J Impot Res. 2004. 16:403–411.
Article30. Lin CS, Ho HC, Chen KC, Lin G, Nunes L, Lue TF. Intracavernosal injection of vascular endothelial growth factor induces nitric oxide synthase isoforms. BJU Int. 2002. 89:955–960.
Article31. Byrne RR, Henry GD, Rao DS, Huynh TT, Pippen AM, Annex BH, et al. Vascular endothelial growth factor restores corporeal smooth muscle function in vitro. J Urol. 2001. 165:1310–1315.
Article32. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. New Engl J Med. 1994. 331:1286–1292.33. Moreland RB, Huang YH, Munnariz R, Nehra A, Goldstein I, Saenz de Tejada I. Oxygen tension regulates the expression of transforming growth factor b1 in human corpus cavernosum smooth muscle. J Urol. 1995. 153:509.34. Moreland RB, Traish AM, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-b1 in human corpus cavernosum smooth muscle. J Urol. 1995. 153:826–834.
Article35. Ryu JK, Han JY, Chu YC, Song SU, Lee KH, Yoon SM, et al. Expression of cavernous transforming growth factor-beta1 and its type II receptor in patients with erectile dysfunction. Int J Androl. 2004. 27:42–49.
Article36. Nehra A, Gettman MT, Nugent M, Bostwick DG, Barrett DM, Goldstein I, et al. Transforming growth factor-beta1 (TGF-beta1) is sufficient to induce fibrosis of rabbit corpus cavernosum in vivo. J Urol. 1999. 162:910–915.
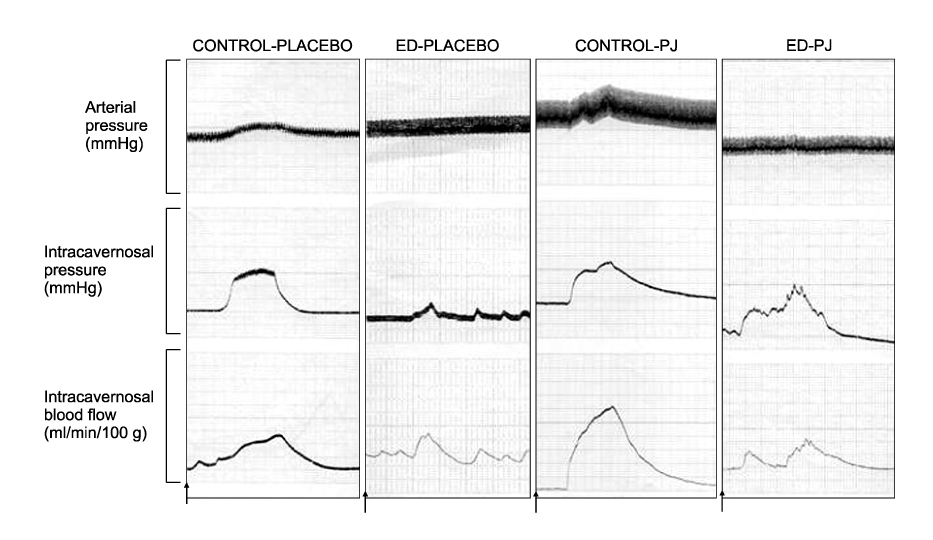
Article37. Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J Urol. 2005. 174:386–393.
Article38. Chade AR, Krier JD, Rodriquez-Porcel M, Breen JF, McKusick MA, Lerman A, et al. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol. 2004. 286:F1079–F1086.
Article39. Mangoush O, Nakamura K, Al-Ruzzeh S, Athanasiou T, Chester A, Amrani M. Effect of ascorbic acid on endothelium-dependent vasodilatation of human arterial conduits for coronary artery bypass grafting. Eur J Cardiothorac Surg. 2003. 24:541–546.
Article40. Fitzpatrick DF, Bing B, Rohdewald P. Endothelium-dependent vascular effects of Pycnogenol. J Cardiovasc Pharmacol. 1998. 32:509–515.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of perineal muscle stimulation by vibration to intracavernous pressure
- Evaluation of Erectile Dysfunction by Penile Duplex Doppler Ultrasonography: Trimix Intracavernosal Injection Versus Vardenafil Oral Medication
- Ultrastructural changes of corporeal smooth muscle cells invasculogenic impotence
- Early Experience of Penile Revascularization Procedure with Anastomosis of Inferior Epigastric Artery to Dorsal Penile Artery in Arteriogenic Impotent Patients
- Inhibition of MicroRNA-92a Improved Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats via Suppressing Oxidative Stress and Endothelial Dysfunction