Cancer Res Treat.
2010 Mar;42(1):42-47.
Maspin Suppresses Survival of Lung Cancer Cells through Modulation of Akt Pathway
- Affiliations
-
- 1Samsung Biomedical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. cpark@skku.edu
Abstract
- PURPOSE
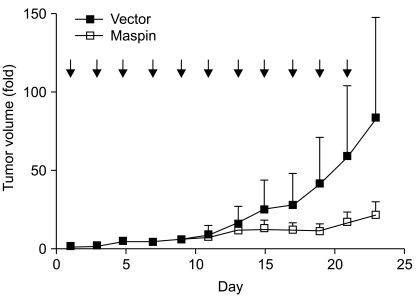
Maspin is a tumor suppressor protein that has been reported to stimulate the cell death of cancer and inhibit the metastasis of cancer. The present study aimed to explore the survival pathway by which maspin modulates the resistance of human lung cancer cells to chemotherapeutic drugs, and the consequences of maspin gene therapy in an animal model. MATERIALS AND METHODS: NCI-H157 and A549 cells were transfected with either a mock vector (pCMVTaq4C), maspin (pCMV-maspin), siControl or siMaspin. RT-PCR and Western blot analysis were performed to study the expressions of survival proteins in lung cancer. cDNA microarray analysis was carried out to compare the maspin-modulated gene expression between the xenograft tumors derived from the lung cancer cells that were stably transfected with pCMVTaq4C or pCMV-maspin. Maspin gene therapy was performed by intra-tumoral injections of pCMVTaq4C or pCMV-maspin into the pre-established subcutaneous tumors in nude mice. RESULTS: Maspin significantly decreased the survival to doxorubicin and etoposide, whereas did not affect the survival to cisplatin in the NCI-H157 cells. Interestingly, transfection with a maspin plasmid resulted in a significant reduction of the phosphorylation of Akt in the NCI-H157 cells, whereas knockdown of maspin increased the phosphorylation of Akt in the A549 cells. Microarray analysis of the xenograft tumors revealed a specific gene expression profile, demonstrating that maspin is associated with the differential expressions of PTEN and IGF2R. Direct transfer of pCMV-maspin into the tumor significantly retarded the tumor growth in the animal experiments (p=0.0048). CONCLUSION: Lung cancer cells lacking maspin could be resistant to chemotherapeutic drugs such as doxorubicin or etoposide, at least in part by maintaining Akt phosphorylation.
Keyword
MeSH Terms
-
Animal Experimentation
Animals
Blotting, Western
Cell Death
Cisplatin
Doxorubicin
Etoposide
Gene Expression
Genetic Therapy
Humans
Lung
Lung Neoplasms
Mice
Mice, Nude
Microarray Analysis
Models, Animal
Neoplasm Metastasis
Oligonucleotide Array Sequence Analysis
Phosphorylation
Plasmids
Proteins
Serpins
Transcriptome
Transfection
Transplantation, Heterologous
Cisplatin
Doxorubicin
Etoposide
Proteins
Serpins
Figure
Reference
-
1. Schneider SS, Schick C, Fish KE, Miller E, Pena JC, Treter SD, et al. A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc Natl Acad Sci USA. 1995; 92:3147–3151. PMID: 7724531.
Article2. Sheng S, Pemberton PA, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem. 1994; 269:30988–30993. PMID: 7983035.
Article3. McGowen R, Biliran H Jr, Sager R, Sheng S. The surface of prostate carcinoma DU145 cells mediates the inhibition of urokinase-type plasminogen activator by maspin. Cancer Res. 2000; 60:4771–4778. PMID: 10987285.4. Pemberton PA, Wong DT, Gibson HL, Kiefer MC, Fitzpatrick PA, Sager R, et al. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin. J Biol Chem. 1995; 270:15832–15837. PMID: 7797587.5. Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res. 2004; 64:6900–6905. PMID: 15466179.
Article6. Jang H, Nam ES, Yeom S, Lee KH, Son HJ, Park C. Maspin polymorphism associated with susceptibility to apoptosis and in vivo tumorigenesis. Int J Mol Med. 2008; 22:333–338. PMID: 18698492.7. Liu J, Yin S, Reddy N, Spencer C, Sheng S. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res. 2004; 64:1703–1711. PMID: 14996730.
Article8. Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994; 263:526–529. PMID: 8290962.
Article9. Hendrix MJ. De-mystifying the mechanism(s) of maspin. Nat Med. 2000; 6:374–376. PMID: 10742136.
Article10. Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996; 93:11669–11674. PMID: 8876194.
Article11. Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001; 114:2903–2910. PMID: 11686294.12. Kang HC, Kim IJ, Park JH, Shin Y, Ku JL, Jung MS, et al. Identification of genes with differential expression in acquired drug-resistant gastric cancer cells using high-density oligonucleotide microarrays. Clin Cancer Res. 2004; 10:272–284. PMID: 14734480.
Article13. Moorehead RA, Singh G. Influence of the proto-oncogene c-fos on cisplatin sensitivity. Biochem Pharmacol. 2000; 59:337–345. PMID: 10644041.
Article14. Hettinga JV, Lemstra W, Meijer C, Los G, de Vries EG, Konings AW, et al. Heat-shock protein expression in cisplatin-sensitive and -resistant human tumor cells. Int J Cancer. 1996; 67:800–807. PMID: 8824551.
Article15. Mizutani Y, Fukumoto M, Bonavida B, Yoshida O. Enhancement of sensitivity of urinary bladder tumor cells to cisplatin by c-myc antisense oligonucleotide. Cancer. 1994; 74:2546–2554. PMID: 7923012.
Article16. Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer. 2000; 82:436–440. PMID: 10646901.
Article17. Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on gastric cancer cell death. Carcinogenesis. 2001; 22:1727–1731. PMID: 11577016.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of the interaction between Hippo/YAP and Akt signaling with ursolic acid and 3′3-diindolylmethane suppresses esophageal cancer tumorigenesis
- Clinicopathological Significance of Maspin Expression in Breast Cancer
- cAMP signaling increases histone deacetylase 8 expression via the Epac2–Rap1A–Akt pathway in H1299 lung cancer cells
- Expression of Maspin in Squamous Cell Carcinoma of the Head and Neck
- Maspin Expression and Its Clinical Significance in Non-Small Cell Lung Cancer