Ann Dermatol.
2016 Jun;28(3):352-359. 10.5021/ad.2016.28.3.352.
Ampelopsis japonica Makino Extract Inhibits the Inflammatory Reaction Induced by Pathogen-Associated Molecular Patterns in Epidermal Keratinocytes
- Affiliations
-
- 1Department of Dermatology, Chungnam National University School of Medicine, Daejeon, Korea. jhoon@cnu.ac.kr
- 2Department of Obstetrics and Gynecology, Dongguk University College of Korean Medicine, Seoul, Korea.
- 3Skin Med Company, Daejeon, Korea.
- KMID: 2164643
- DOI: http://doi.org/10.5021/ad.2016.28.3.352
Abstract
- BACKGROUND
Keratinocytes are the major cells in epidermis, providing barrier components such as cornified cells through the sophisticated differentiation process. In addition, keratinocytes exerts their role as the defense cells via activation of innate immunity. It has been known that pathogen-associated molecular patterns (PAMPs) including double-strand RNA and nucleotides can provoke inflammatory reaction in keratinocytes.
OBJECTIVE
The aim of this study is to evaluate the effect of Ampelopsis japonica Makino extract (AE) on PAMPs-induced inflammatory reaction of keratinocytes.
METHODS
The effects of AE were determined using poly (I:C)-induced inflammation and imiquimod-induced psoriasiform dermatitis models.
RESULTS
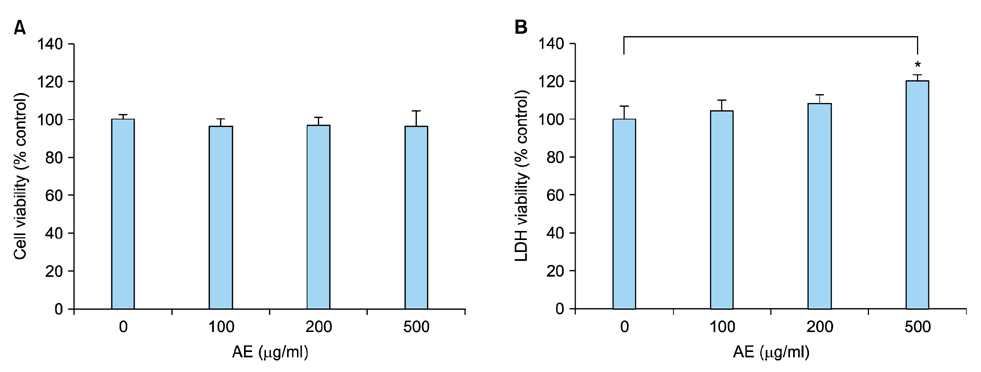
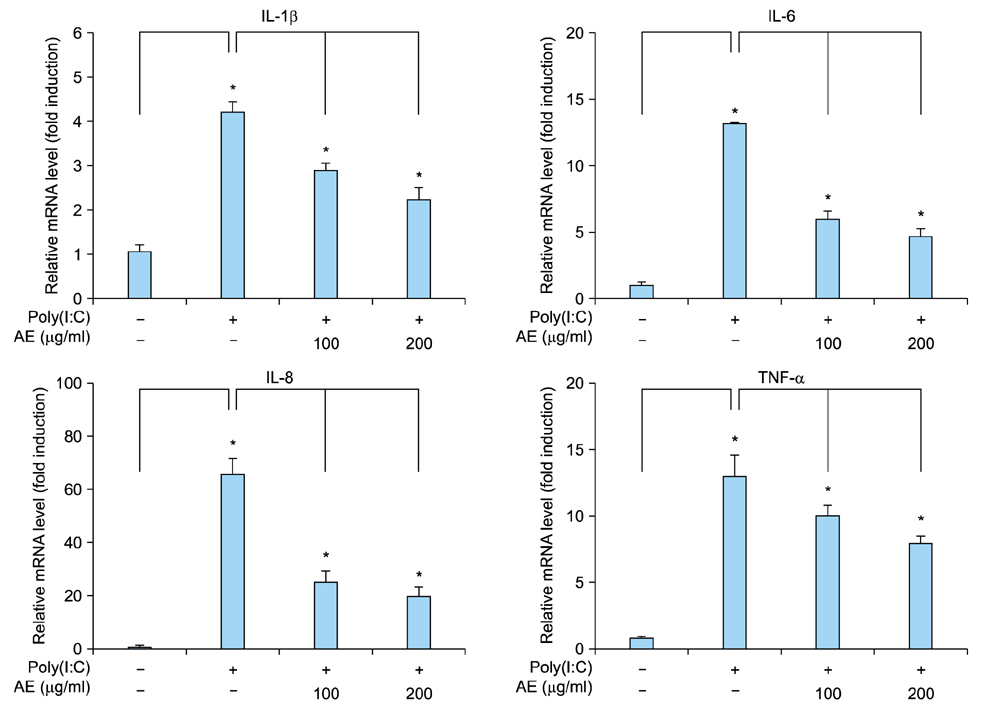
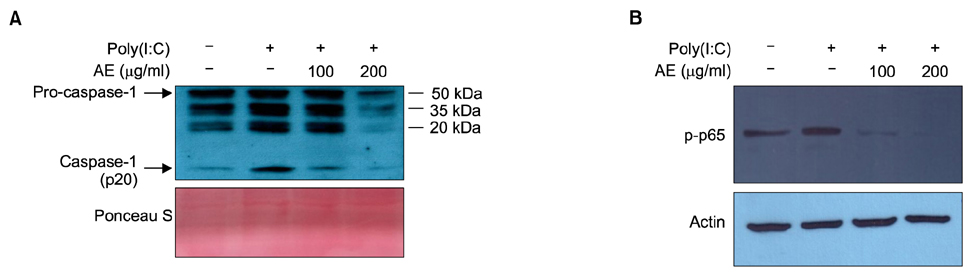
In cultured keratinocytes, AE significantly inhibited poly(I:C)-induced expression of inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor-α. AE significantly inhibited poly(I:C)-induced release of caspase-1 active form (p20), and down-regulated nuclear factor-κB signaling pathway. In imiquimod-induced psoriasiform dermatitis model, topical application of AE resulted in significant reduction of epidermal hyperplasia.
CONCLUSION
These results suggest that AE may be a potential candidate for the treatment of skin inflammation.
MeSH Terms
-
Ampelopsis*
Cytokines
Dermatitis
Epidermis
Hyperplasia
Immunity, Innate
Inflammation
Interleukin-6
Interleukin-8
Interleukins
Keratinocytes*
Necrosis
Nucleotides
Pathogen-Associated Molecular Pattern Molecules*
RNA
Skin
Cytokines
Interleukin-6
Interleukin-8
Interleukins
Nucleotides
Pathogen-Associated Molecular Pattern Molecules
RNA
Figure
Reference
-
1. Stalder JF, Tennstedt D, Deleuran M, Fabbrocini G, de Lucas R, Haftek M, et al. Fragility of epidermis and its consequence in dermatology. J Eur Acad Dermatol Venereol. 2014; 28:Suppl 4. 1–18.
Article2. Rice RH, Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977; 11:417–422.
Article3. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995; 270:17702–17711.
Article4. Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999; 31:5–19.
Article5. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001; 1:135–145.
Article6. McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005; 125:1–8.
Article7. Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007; 7:179–190.
Article8. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007; 445:866–873.
Article9. Valdimarsson H, Bake BS, Jónsdótdr I, Fry L. Psoriasis: a disease of abnormal Keratinocyte proliferation induced by T lymphocytes. Immunol Today. 1986; 7:256–259.
Article10. Lebre MC, van der, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007; 127:331–341.
Article11. Prens EP, Kant M, van Dijk G, van der Wel LI, Mourits S, van der Fits L. IFN-alpha enhances poly-IC responses in human keratinocytes by inducing expression of cytosolic innate RNA receptors: relevance for psoriasis. J Invest Dermatol. 2008; 128:932–938.
Article12. Chen X, Takai T, Xie Y, Niyonsaba F, Okumura K, Ogawa H. Human antimicrobial peptide LL-37 modulates proinflammatory responses induced by cytokine milieus and double-stranded RNA in human keratinocytes. Biochem Biophys Res Commun. 2013; 433:532–537.
Article13. Nho KJ, Chun JM, Kim DS, Kim HK. Ampelopsis japonica ethanol extract suppresses migration and invasion in human MDA-MB-231 breast cancer cells. Mol Med Rep. 2015; 11:3722–3728.
Article14. Andrés RM, Montesinos MC, Navalón P, Payá M, Terencio MC. NF-κB and STAT3 inhibition as a therapeutic strategy in psoriasis: in vitro and in vivo effects of BTH. J Invest Dermatol. 2013; 133:2362–2371.
Article15. Grimstad Ø, Husebye H, Espevik T. TLR3 mediates release of IL-1β and cell death in keratinocytes in a caspase-4 dependent manner. J Dermatol Sci. 2013; 72:45–53.
Article16. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836–5845.
Article17. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002; 3:196–200.
Article18. Sweeney CM, Tobin AM, Kirby B. Innate immunity in the pathogenesis of psoriasis. Arch Dermatol Res. 2011; 303:691–705.
Article19. Kim IH, Umezawa M, Kawahara N, Goda Y. The constituents of the roots of Ampelopsis japonica. J Nat Med. 2007; 61:224–225.
Article20. Park H, Shim JS, Kim HG, Lee H, Oh MS. Ampelopsis radix protects dopaminergic neurons against 1-methyl-4-phenylpyridinium/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-In duced toxicity in Parkinson's disease models in vitro and in vivo. Evid Based Complement Alternat Med. 2013; 2013:346438.21. Kürbitz C, Heise D, Redmer T, Goumas F, Arlt A, Lemke J, et al. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and antiinflammatory activities in pancreatic tumor cells. Cancer Sci. 2011; 102:728–734.
Article22. Nakanishi T, Mukai K, Yumoto H, Hirao K, Hosokawa Y, Matsuo T. Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteria-derived factors. Eur J Oral Sci. 2010; 118:145–150.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Ampelopsis japonica Makino Extract Inhibits the Inflammatory Reaction Induced by Pathogen-Associated Molecular Patterns in Epidermal Keratinocytes
- Aster glehni Ethanol Extract Inhibits Inflammatory Responses Regulating Skin Barrier Molecules in Human Keratinocytes
- Rhododendrin inhibits toll-like receptor-7-mediated psoriasis-like skin inflammation in mice
- Immunomodulatory Effects of Callophyllis japonica Ethanol Extract on Dendritic Cells
- AllogeneicLymphocyte Stimulating Capacity of Contact Sensitized Epidermal Cells in Mouse