Immune Netw.
2011 Apr;11(2):107-113. 10.4110/in.2011.11.2.107.
Dietary Aloe Reduces Adipogenesis via the Activation of AMPK and Suppresses Obesity-related Inflammation in Obese Mice
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2Department of Food and Nutrition, Seoul National University, Seoul 151-742, Korea.
- 3Univera Inc., Seoul 133-120, Korea.
- 4School of Life Sciences and Biotechnology, Korea University, Seoul 136-701, Korea.
- 5College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 2150698
- DOI: http://doi.org/10.4110/in.2011.11.2.107
Abstract
- BACKGROUND
Metabolic disorders, including type II diabetes and obesity, present major health risks in industrialized countries. AMP-activated protein kinase (AMPK) has become the focus of a great deal of attention as a novel therapeutic target for the treatment of metabolic syndromes. In this study, we evaluated whether dietary aloe could reduce obesity-induced inflammation and adipogenesis.
METHODS
Male C57BL/6 obese mice fed a high-fat diet for 54 days received a supplement of aloe formula (PAG, ALS, Aloe QDM, and Aloe QDM complex) or pioglitazone (PGZ) and were compared with unsupplemented controls (high-fat diet; HFD) or mice fed a regular diet (RD). RT-PCR and western blot analysis were used to quantify the expression of obesity-induced inflammation.
RESULTS
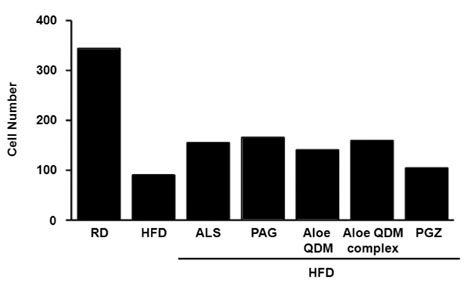
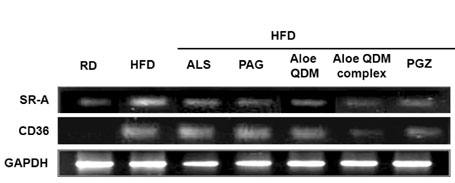
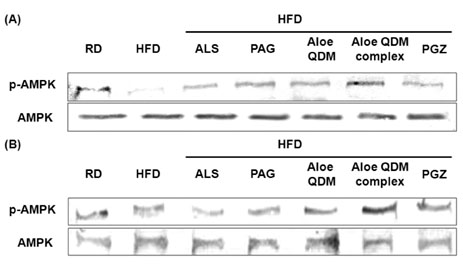
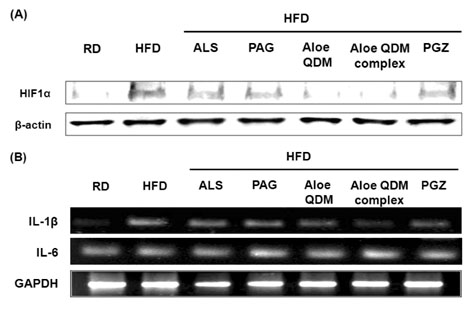
Aloe QDM complex down-regulated fat size through suppressed expression of scavenger receptors on adipose tissue macrophages (ATMs) compared with HFD. Both white adipose tissue (WATs) and muscle exhibited increased AMPK activation through aloe supplementation, and in particular, the Aloe QDM complex. Obesity-induced inflammatory cytokines (IL-1beta and -6) and HIF1alpha mRNA and protein were decreased markedly, as was macrophage infiltration by the Aloe QDM complex. Further, the Aloe QDM complex decreased the translocation of NF-kappaB p65 from the cytosol in the WAT.
CONCLUSION
Dietary aloe formula reduced obesity-induced inflammatory responses by activation of AMPK in muscle and suppression of proinflammatory cytokines in the WAT. Additionally, the expression of scavenger receptors in the ATM and activation of AMPK in WAT led to reduction in the percent of body fat. Thus, we suggest that the effect of the Aloe QDM complex in the WAT and muscle are related to activation of AMPK and its use as a nutritional intervention against T2D and obesity-related inflammation.
MeSH Terms
-
Adipogenesis
Adipose Tissue
Adipose Tissue, White
Aloe
AMP-Activated Protein Kinases
Animals
Blotting, Western
Cytokines
Cytosol
Developed Countries
Diabetes Mellitus, Type 2
Diet
Diet, High-Fat
Humans
Inflammation
Macrophages
Male
Mice
Mice, Obese
Muscles
NF-kappa B
Obesity
Receptors, Scavenger
RNA, Messenger
Thiazolidinediones
AMP-Activated Protein Kinases
Cytokines
NF-kappa B
RNA, Messenger
Receptors, Scavenger
Thiazolidinediones
Figure
Cited by 1 articles
-
The Synergy Effect of Weight‐Bearing Circuit Training and Aloe QDM Complex on Obese Middle Aged Women: a Randomized Double‐Blind Controlled Trial
Mi Jung Choi, Yong An Kim, Eunju Shin, Seon-Gil Do, Wook Song
Korean J Health Promot. 2014;14(2):59-66. doi: 10.15384/kjhp.2014.14.2.59.
Reference
-
1. Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003. 144:3765–3773.
Article2. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005. 115:1111–1119.
Article3. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003. 112:1796–1808.
Article4. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003. 112:1821–1830.
Article5. Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997. 246:259–273.
Article6. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008. 181:8633–8641.
Article7. Makinde AO, Gamble J, Lopaschuk GD. Upregulation of 5'-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res. 1997. 80:482–489.
Article8. Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002. 99:15983–15987.
Article9. Ju JS, Gitcho MA, Casmaer CA, Patil PB, Han DG, Spencer SA, Fisher JS. Potentiation of insulin-stimulated glucose transport by the AMP-activated protein kinase. Am J Physiol Cell Physiol. 2007. 292:C564–C572.
Article10. Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002. 51:2886–2894.
Article11. Shin E, Shim KS, Kong H, Lee S, Shin S, Kwon J, Jo TH, Park YI, Lee CK, Kim K. Dietary Aloe Improves Insulin Sensitivity via the Suppression of Obesity-induced Inflammation in Obese Mice. Immune Netw. 2011. 11:59–67.
Article12. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001. 409:307–312.
Article13. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005. 436:356–362.
Article14. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010. 72:219–246.
Article15. Mertz W, Schwarz K. Relation of glucose tolerance factor to impaired intravenous glucose tolerance of rats on stock diets. Am J Physiol. 1959. 196:614–618.
Article16. Schwarz K, Mertz W. Chromium(III) and the glucose tolerance factor. Arch Biochem Biophys. 1959. 85:292–295.
Article17. Anderson RA. Recent advances in the clinical and biochemical effects of chromium deficiency. Prog Clin Biol Res. 1993. 380:221–234.18. Mertz W. Chromium in human nutrition: a review. J Nutr. 1993. 123:626–633.
Article19. Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr. 1998. 17:548–555.
Article20. Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, Lee YH, Lee YR, Oh ST, Jo TH, Park YI, Lee CK, Kim K. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009. 16:856–863.
Article21. Kong HS, Lee SW, Shin SM, Kwon JH, Jo TH, Shin EJ, Shim KS, Park YI, Lee CK, Kim KJ. Down-regulation of adipogenesis and hyperglycemia in diet-induced obesity mouse model by aloe QDM. Biomol Ther. 2010. 18:336–342.
Article22. Kim JO, Kim KS, Lee GD, Kwon JH. Antihyperglycemic and antioxidative effects of new herbal formula in streptozotocin-induced diabetic rats. J Med Food. 2009. 12:728–735.
Article23. Martín-Fuentes P, Civeira F, Recalde D, García-Otín AL, Jarauta E, Marzo I, Cenarro A. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J Immunol. 2007. 179:3242–3248.
Article24. Sahoo D, Drover V. The role of scavenger receptors in signaling, inflammation and atherosclerosis. Biochemistry of Atherosclerosis. 2006. 1:70–91.
Article25. Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001. 3:149–159.
Article26. Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010. 11:155–161.
Article27. Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zähringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005. 433:523–527.
Article28. Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005. 170:477–485.
Article29. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006. 116:3015–3025.
Article30. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007. 56:1986–1998.
Article31. Regazzetti C, Peraldi P, Grémeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009. 58:95–103.
Article32. Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008. 100:227–235.
Article33. Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005. 54:2277–2286.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dietary Aloe QDM Complex Reduces Obesity-Induced Insulin Resistance and Adipogenesis in Obese Mice Fed a High-Fat Diet
- Dietary Aloe Improves Insulin Sensitivity via the Suppression of Obesity-induced Inflammation in Obese Mice
- Capsanthin Inhibits both Adipogenesis in 3T3-L1 Preadipocytes and Weight Gain in High-Fat Diet-Induced Obese Mice
- Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet
- Quercetin Upregulates Uncoupling Protein 1 in White/Brown Adipose Tissues through Sympathetic Stimulation