Immune Netw.
2012 Jun;12(3):96-103. 10.4110/in.2012.12.3.96.
Dietary Aloe QDM Complex Reduces Obesity-Induced Insulin Resistance and Adipogenesis in Obese Mice Fed a High-Fat Diet
- Affiliations
-
- 1College of Pharmacy, SahmYook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2Univera Inc., Seoul 133-120, Korea.
- 3School of Life Sciences and Biotechnology, Korea University, Seoul 136-701, Korea.
- 4College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 2168007
- DOI: http://doi.org/10.4110/in.2012.12.3.96
Abstract
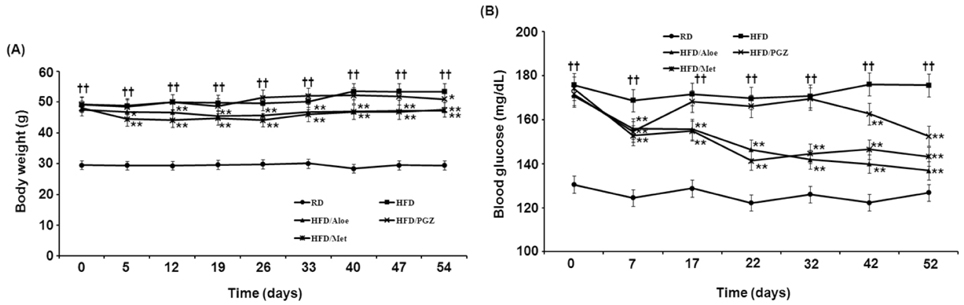
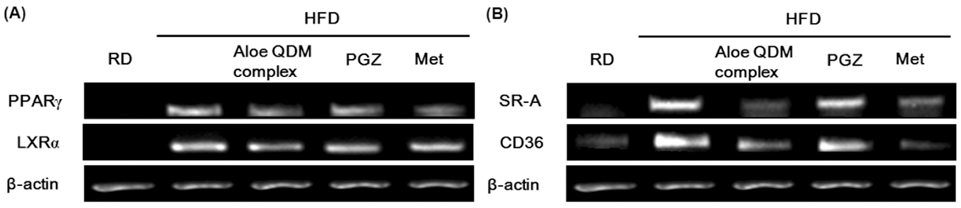
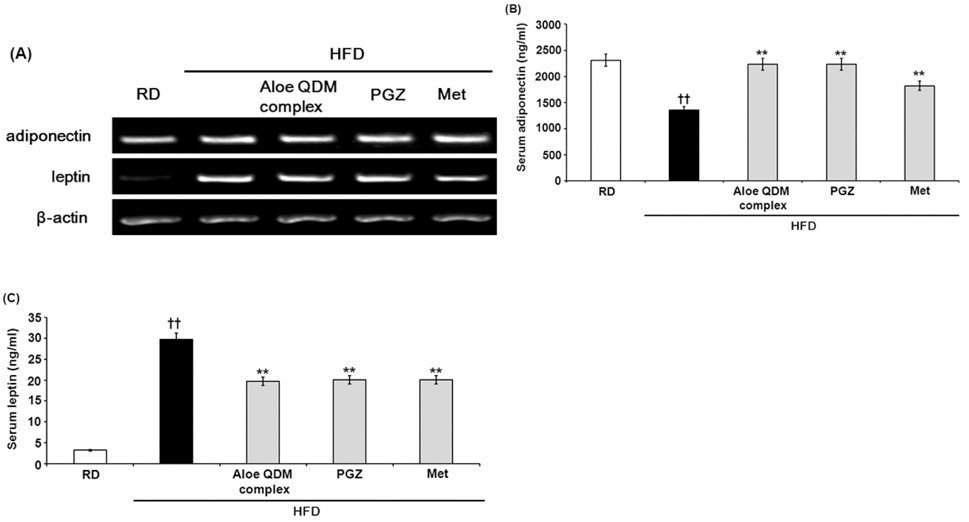
- Obesity-induced disorders contribute to the development of metabolic diseases such as insulin resistance, fatty liver diseases, and type 2 diabetes (T2D). In this study, we evaluated whether the Aloe QDM complex could improve metabolic disorders related to blood glucose levels and insulin resistance. Male C57BL/6 obese mice fed a high-fat diet for 54 days received a supplement of Aloe QDM complex or pioglitazone (PGZ) or metformin (Met) and were compared with unsupplemented controls (high-fat diet; HFD) or mice fed a regular diet (RD). RT-PCR and western blot analysis were used to quantify the expression of obesity-induced inflammation. Dietary Aloe QDM complex lowered body weight, fasting blood glucose, plasma insulin, and leptin levels, and markedly reduced the impairment of glucose tolerance in obese mice. Also, Aloe QDM complex significantly enhanced plasma adiponectin levels and insulin sensitivity via AMPK activity in muscles. At the same time, Aloe QDM decreased the mRNA and protein of PPARgamma/LXRalpha and scavenger receptors in white adipose tissue (WAT). Dietary Aloe QDM complex reduces obesity-induced glucose tolerance not only by suppressing PPARgamma/LXRalpha but also by enhancing AMPK activity in the WAT and muscles, both of which are important peripheral tissues affecting insulin resistance. The Aloe QDM complex could be used as a nutritional intervention against T2D.
MeSH Terms
-
Adipogenesis
Adiponectin
Adipose Tissue, White
Aloe
Animals
Blood Glucose
Blotting, Western
Body Weight
Diabetes Mellitus, Type 2
Diet
Diet, High-Fat
Fasting
Fatty Liver
Glucose
Humans
Inflammation
Insulin
Insulin Resistance
Leptin
Male
Metabolic Diseases
Metformin
Mice
Mice, Obese
Muscles
Plasma
Receptors, Scavenger
RNA, Messenger
Thiazolidinediones
Adiponectin
Blood Glucose
Glucose
Insulin
Leptin
Metformin
RNA, Messenger
Receptors, Scavenger
Thiazolidinediones
Figure
Reference
-
1. Weiser M, Frishman WH, Michaelson MD, Abdeen MA. The pharmacologic approach to the treatment of obesity. J Clin Pharmacol. 1997. 37:453–473.
Article2. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988. 37:1163–1167.
Article3. Stunkard AJ. Current views on obesity. Am J Med. 1996. 100:230–236.
Article4. Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009. 53:508–515.
Article5. Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L'Abbate A, Abraham NG. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009. 50:1293–1304.
Article6. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008. 8:224–236.
Article7. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005. 115:1111–1119.
Article8. Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003. 144:3765–3773.
Article9. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003. 112:1796–1808.
Article10. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003. 112:1821–1830.
Article11. Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997. 246:259–273.
Article12. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008. 181:8633–8641.
Article13. Makinde AO, Gamble J, Lopaschuk GD. Upregulation of 5'-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res. 1997. 80:482–489.
Article14. Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002. 99:15983–15987.
Article15. Ju JS, Gitcho MA, Casmaer CA, Patil PB, Han DG, Spencer SA, Fisher JS. Potentiation of insulin-stimulated glucose transport by the AMP-activated protein kinase. Am J Physiol Cell Physiol. 2007. 292:C564–C572.
Article16. Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002. 51:2886–2894.
Article17. Josep BR, Amir G, Jennifer K, Raquel H. Peroxisome proliferator activated receptors: the nutritionally controlled molecular networks that integrate inflammation, immunity and metabolism. Current Nutrition & Food Science. 2005. 1:179–187.18. Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007. 47:185–210.
Article19. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998. 67:821–855.
Article20. Capasso F, Borrelli F, Capasso R, Di Carlo G, Izzo A, Pinto L, Mascolo N, Castaldo S, Longo R. Aloe and its therapeutic use. Phytother Res. 1998. 12:S124–S127.
Article21. Heggers JP, Kucukcelebi A, Stabenau CJ, Ko F, Broemeling LD, Robson MC, Winters WD. Wound healing effects of Aloe gel and other topical antibacterial agents on rat skin. Phytother Res. 1995. 9:455–457.
Article22. Koo MWL. Aloe vera: Antiulcer and antidiabetic effects. Phytother Res. 1994. 8:461–464.
Article23. Winters WD, Benavides R, Clouse WJ. Effects of aloe extracts on human normal and tumor cells in vitro. Econ Bot. 1981. 35:89–95.
Article24. Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996. 3:241–243.
Article25. Bunyapraphatsara N, Yongchaiyudha S, Rungpitarangsi V, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice: II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine. 1996. 3:245–248.
Article26. Kong H, Lee S, Shin S, Kwon J, Jo TH, Shin E, Shim KS, Park YI, Lee CK, Kim K. Down-regulation of adipogenesis and hyperglycemia in diet-induced obesity mouse model by Aloe QDM. Biomolecules & Therapeutics. 2010. 18:336–342.
Article27. Kim JO, Kim KS, Lee GD, Kwon JH. Antihyperglycemic and antioxidative effects of new herbal formula in streptozotocin-induced diabetic rats. J Med Food. 2009. 12:728–735.
Article28. Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, Lee YH, Lee YR, Oh ST, Jo TH, Park YI, Lee CK, Kim K. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009. 16:856–863.
Article29. Martín-Fuentes P, Civeira F, Recalde D, García-Otín AL, Jarauta E, Marzo I, Cenarro A. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J Immunol. 2007. 179:3242–3248.
Article30. Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010. 11:155–161.
Article31. Sahoo D, Drover V. The role of scavenger receptors in signaling, inflammation and atherosclerosis. Biochemistry of Atherosclerosis. 2006. 1:70–91.
Article32. Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001. 3:149–159.
Article33. Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010. 11:155–161.
Article34. Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zähringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005. 433:523–527.
Article35. Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005. 170:477–485.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dietary Aloe Improves Insulin Sensitivity via the Suppression of Obesity-induced Inflammation in Obese Mice
- Dietary Aloe Reduces Adipogenesis via the Activation of AMPK and Suppresses Obesity-related Inflammation in Obese Mice
- The Synergy Effect of Weight-Bearing Circuit Training and Aloe QDM Complex on Obese Middle Aged Women: a Randomized Double-Blind Controlled Trial
- Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice
- Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice