Curcumin Induces Downregulation of E2F4 Expression and Apoptotic Cell Death in HCT116 Human Colon Cancer Cells; Involvement of Reactive Oxygen Species
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Catholic University of Daegu, Daegu 705-718, Korea.
- 2Department of Biochemistry and Molecular Biology, School of Medicine, Yeungnam University, Daegu 705-717, Korea. chlee2@yun.ac.kr
- 3Aging-Associated Vascular Disease Research Center, School of Medicine, Yeungnam University, Daegu 705-717, Korea.
- KMID: 2071711
- DOI: http://doi.org/10.4196/kjpp.2010.14.6.391
Abstract
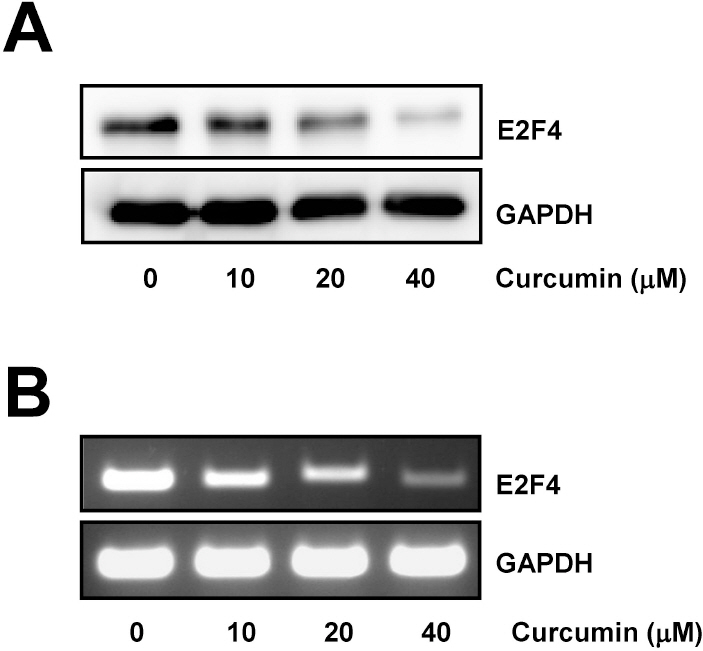
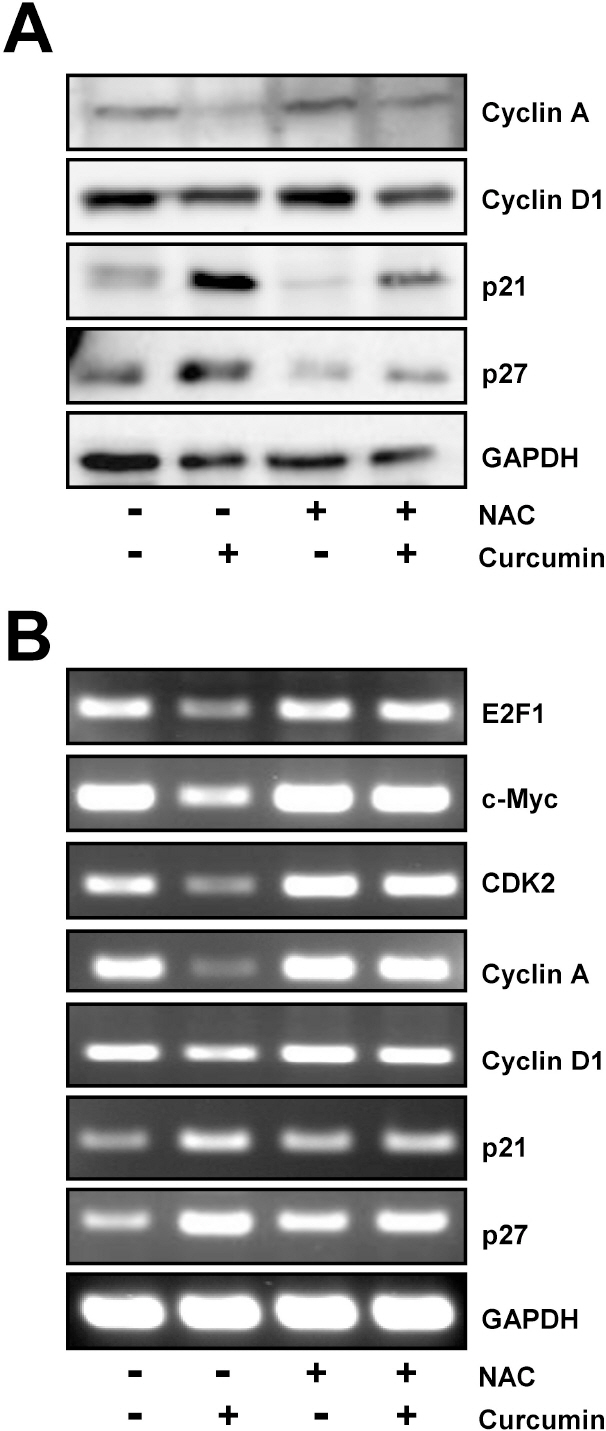
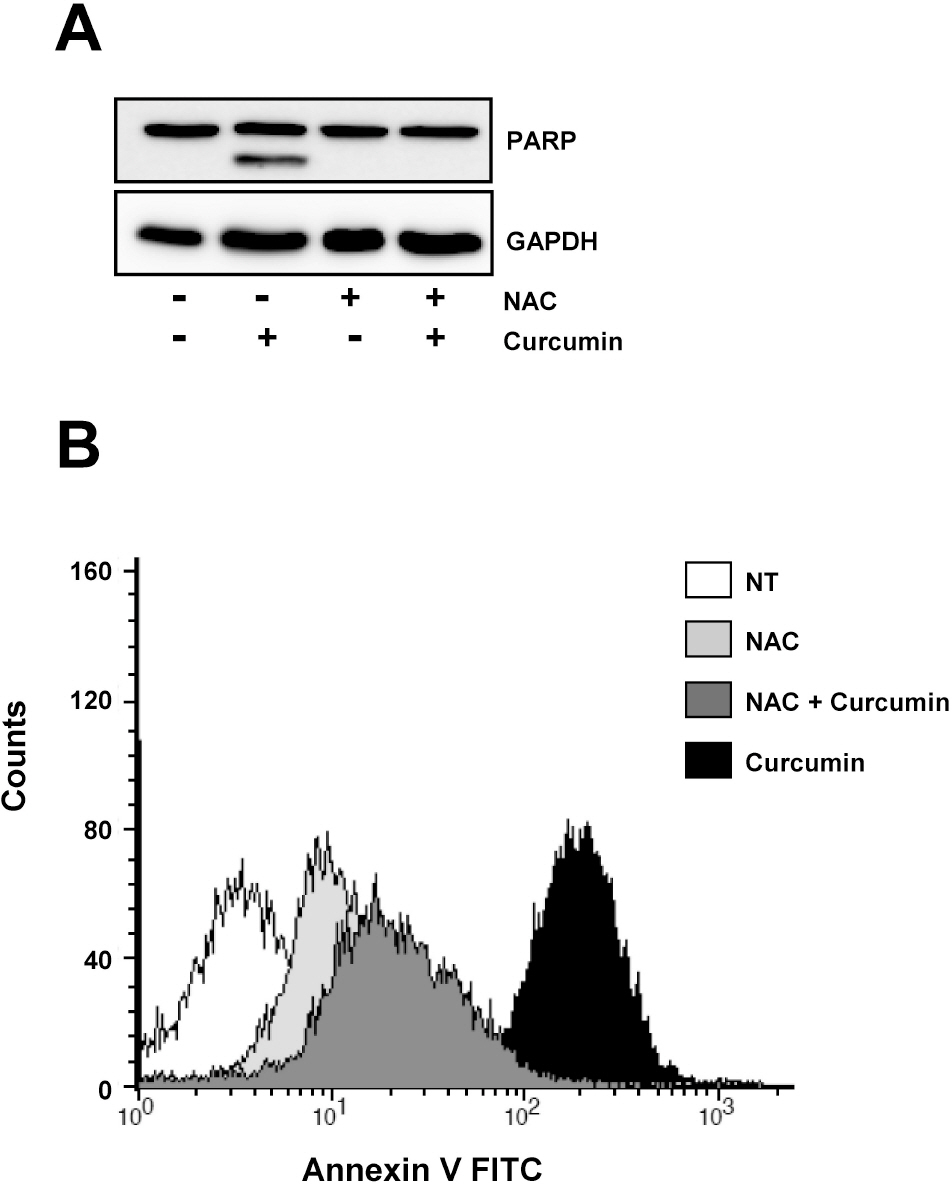
- E2F transcription factors and their target genes have been known to play an important role in cell growth control. We found that curcumin, a polyphenolic phytochemical isolated from the plant Curcuma longa, markedly suppressed E2F4 expression in HCT116 colon cancer cells. Hydrogen peroxide was also found to decrease E2F4 protein level, indicating the involvement of reactive oxygen species (ROS) in curucmin-induced downregulation of E2F4 expression. Involvement of ROS in E2F4 downregulation in response to curcumin was confirmed by the result that pretreatment of cells with N-acetylcystein (NAC) before exposure of curcumin almost completely blocked the reduction of E2F4 expression at the protein as well as mRNA level. Anti-proliferative effect of curcumin was also suppressed by NAC which is consistent to previous reports showing curcumin-superoxide production and induction of poly (ADP-ribose) polymerase (PARP) cleavage as well as apoptosis. Expression of several genes, cyclin A, p21, and p27, which has been shown to be regulated in E2F4-dependent manner and involved in the cell cycle progression was also affected by curcumin. Moreover, decreased (cyclin A) and increased (p21 and p27) expression of these E2F4 downstream genes by curcumin was restored by pretreatment of cells with NAC and E2F4 overexpression which is induced by doxycycline. In addition, E2F4 overexpression was observed to partially ameliorate curcumin-induced growth inhibition by cell viability assay. Taken together, we found curcumin-induced ROS down-regulation of E2F4 expression and modulation of E2F4 target genes which finally lead to the apoptotic cell death in HCT116 colon cancer cells, suggesting that E2F4 appears to be a novel determinant of curcumin-induced cytotoxicity.
Keyword
MeSH Terms
-
Apoptosis
Cell Cycle
Cell Death
Cell Proliferation
Cell Survival
Colon
Colonic Neoplasms
Curcuma
Curcumin
Cyclin A
Down-Regulation
Doxycycline
E2F Transcription Factors
Humans
Hydrogen Peroxide
Plants
Reactive Oxygen Species
RNA, Messenger
Staphylococcal Protein A
Curcumin
Cyclin A
Doxycycline
E2F Transcription Factors
Hydrogen Peroxide
RNA, Messenger
Reactive Oxygen Species
Staphylococcal Protein A
Figure
Cited by 5 articles
-
Protective effect of butylated hydroxylanisole against hydrogen peroxide-induced apoptosis in primary cultured mouse hepatocytes
Geun Hye Hwang, Yu Jin Jeon, Ho Jae Han, Soo Hyun Park, Kyoung Min Baek, Woochul Chang, Joong Sun Kim, Lark Kyun Kim, You-Mie Lee, Sangkyu Lee, Jong-Sup Bae, Jun-Goo Jee, Min Young Lee
J Vet Sci. 2015;16(1):17-23. doi: 10.4142/jvs.2015.16.1.17.Increased HoxB4 Inhibits Apoptotic Cell Death in Pro-B Cells
Sung-Won Park, Kyung-Jong Won, Yong-Soo Lee, Hye Sun Kim, Yu-Kyung Kim, Hyeon-Woo Lee, Bokyung Kim, Byeong Han Lee, Jin-Hoi Kim, Dong-Ku Kim
Korean J Physiol Pharmacol. 2012;16(4):265-271. doi: 10.4196/kjpp.2012.16.4.265.Anti-Oxidative and Anti-Inflammatory Effects of QGC in Cultured Feline Esophageal Epithelial Cells
Myeong Jae Lee, Hyun Ju Song, Jun Yeong Jeong, Sun Young Park, Uy Dong Sohn
Korean J Physiol Pharmacol. 2013;17(1):81-87. doi: 10.4196/kjpp.2013.17.1.81.Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-α related mechanism
Shuying Tian, Ruixue Guo, Sichen Wei, Yu Kong, Xinliang Wei, Weiwei Wang, Xiaomeng Shi, Hongyu Jiang
Korean J Physiol Pharmacol. 2016;20(2):147-152. doi: 10.4196/kjpp.2016.20.2.147.GS28 Protects Neuronal Cell Death Induced by Hydrogen Peroxide under Glutathione-Depleted Condition
Hwa Ok Lee, Yu Jeong Byun, Kyung-Ok Cho, Seong Yun Kim, Seong-Beom Lee, Ho-Shik Kim, Oh-Joo Kwon, Seong-Whan Jeong
Korean J Physiol Pharmacol. 2011;15(3):149-156. doi: 10.4196/kjpp.2011.15.3.149.
Reference
-
References
1. Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991; 351:494–497.
Article2. Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991; 65:1053–1061.
Article3. Leone G, Nuckolls F, Ishida S, Adams M, Sears R, Jakoi L, Miron A, Nevins JR. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol Cell Biol. 2000; 20:3626–3632.
Article4. He Y, Armanious MK, Thomas MJ, Cress WD. Identification of E2F–3B, an alternative form of E2F–3 lacking a conserved N-terminal region. Oncogene. 2000; 19:3422–3433.
Article5. DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006; 6:739–748.
Article6. Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993; 365:349–352.
Article7. Morkel M, Wenkel J, Bannister AJ, Kouzarides T, Hagemeier C. An E2F-like repressor of transcription. Nature. 1997; 390:567–568.
Article8. Cartwright P, Muller H, Wagener C, Holm K, Helin K. E2F–6: a novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene. 1998; 17:611–623.
Article9. Trimarchi JM, Fairchild B, Verona R, Moberg K, Andon N, Lees JA. E2F–6, a member of the E2F family that can behave as a transcriptional repressor. Proc Natl Acad Sci USA. 1998; 95:2850–2855.
Article10. Koziczak M, Krek W, Nagamine Y. Pocket protein-independent repression of urokinase-type plasminogen activator and plasminogen activator inhibitor 1 gene expression by E2F1. Mol Cell Biol. 2000; 20:2014–2022.
Article11. Garneau H, Paquin MC, Carrier JC, Rivard N. E2F4 expression is required for cell cycle progression of normal intestinal crypt cells and colorectal cancer cells. J Cell Physiol. 2009; 221:350–358.
Article12. Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999; 39:41–47.
Article13. Oyama Y, Masuda T, Nakata M, Chikahisa L, Yamazaki Y, Miura K, Okagawa M. Protective actions of 5′-n-alkylated curcumins on living cells suffering from oxidative stress. Eur J Pharmacol. 1998; 360:65–71.
Article14. Nagano T, Oyama Y, Kajita N, Chikahisa L, Nakata M, Okazaki E, Masuda T. New curcuminoids isolated from Zingiber cassumunar protect cells suffering from oxidative stress: a flow-cytometric study using rat thymocytes and H2O2. Jpn J Pharmacol. 1997; 75:363–370.
Article15. Cho Jy, Kang PJ, Chun W, Moon YO, Park YT, Lim SY, Kim SS. Curcumin attenuates glial cell activation but cannot suppress hippocampal CA3 neuronal cell death in i.c.v. Kanic Acid Injection Model. Korean J Physiol Pharmacol. 2003; 7:307–310.16. Kong PJ, Kwon OY, Han YH, Kim SY, Lee SN, Son HJ, Kim SS. Comparison of Inhibitory Potency of Various Antioxidants on the Activation of BV2 Microglial Cell Lines Induced by LPS. Korean J Physiol Pharmacol. 2007; 11:9–13.17. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or premalignant lesions. Anticancer Res. 2001; 21:2895–2900.18. Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004; 10:6847–6854.19. Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008; 14:4491–4499.
Article20. Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin causes super-oxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010; 297:1–8.
Article21. Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001; 21:873–878.
Article22. Lee DH, Choi HC, Lee KY, Kang YJ. Aprotinin Inhibits Vascular Smooth Muscle Cell Inflammation and Proliferation via Induction of HO-1. Korean J Physiol Pharmacol. 2009; 13:123–129.
Article23. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007; 595:105–125.
Article24. Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003; 24:1199–1208.
Article25. Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001; 15:267–285.
Article26. Young AP, Nagarajan R, Longmore GD. Mechanisms of transcriptional regulation by Rb-E2F segregate by biological pathway. Oncogene. 2003; 22:7209–7217.
Article27. Polager S, Kalma Y, Berkovich E, Ginsberg D. E2Fs upregulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene. 2002; 21:437–446.
Article28. DuPree EL, Mazumder S, Almasan A. Genotoxic stress induces expression of E2F4, leading to its association with p130 in prostate carcinoma cells. Cancer Res. 2004; 64:4390–4393.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Downregulation of Reactive Oxygen Species in Apoptosis
- Involvement of ROS in Curcumin-induced Autophagic Cell Death
- Cellular Effect of Curcumin and Citral Combination on Breast Cancer Cells: Induction of Apoptosis and Cell Cycle Arrest
- IGF-1 induces expression of zinc-finger protein 143 in colon cancer cells through phosphatidylinositide 3-kinase and reactive oxygen species
- Chrysophanic Acid Induces Necrosis but not Necroptosis in Human Renal Cell Carcinoma Caki-2 Cells