Korean J Physiol Pharmacol.
2011 Feb;15(1):1-7.
Involvement of ROS in Curcumin-induced Autophagic Cell Death
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Catholic University of Daegu, Daegu 705-718, Korea.
- 2Department of Biochemistry and Molecular Biology, School of Medicine, Yeungnam University, Daegu 705-717, Korea. chlee2@ynu.ac.kr
- 3Institute for Innovative Cancer Research, Asan Medical Center, Seoul 138-736, Korea.
Abstract
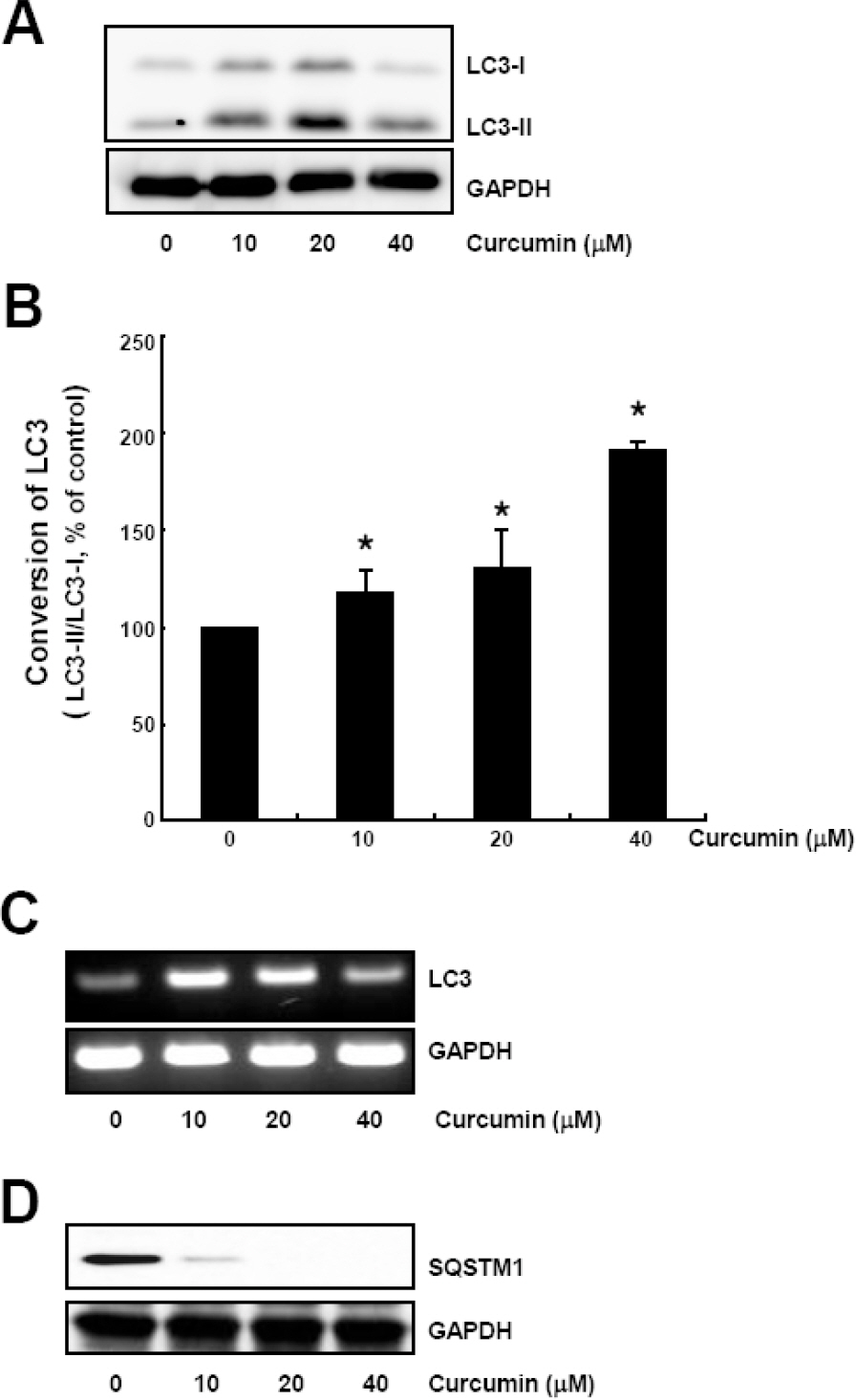
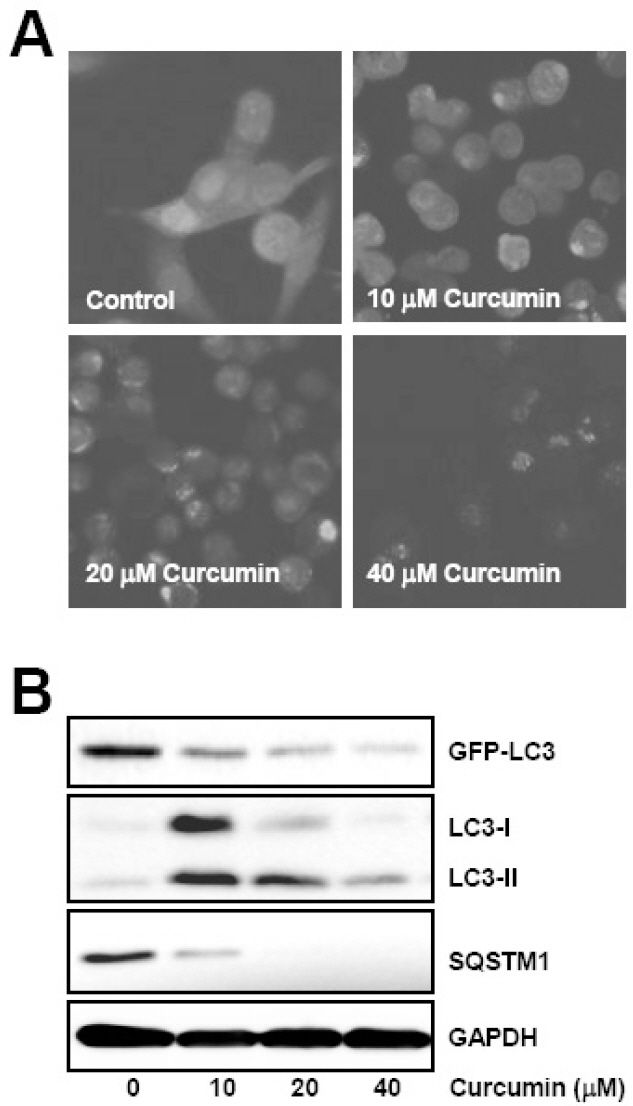
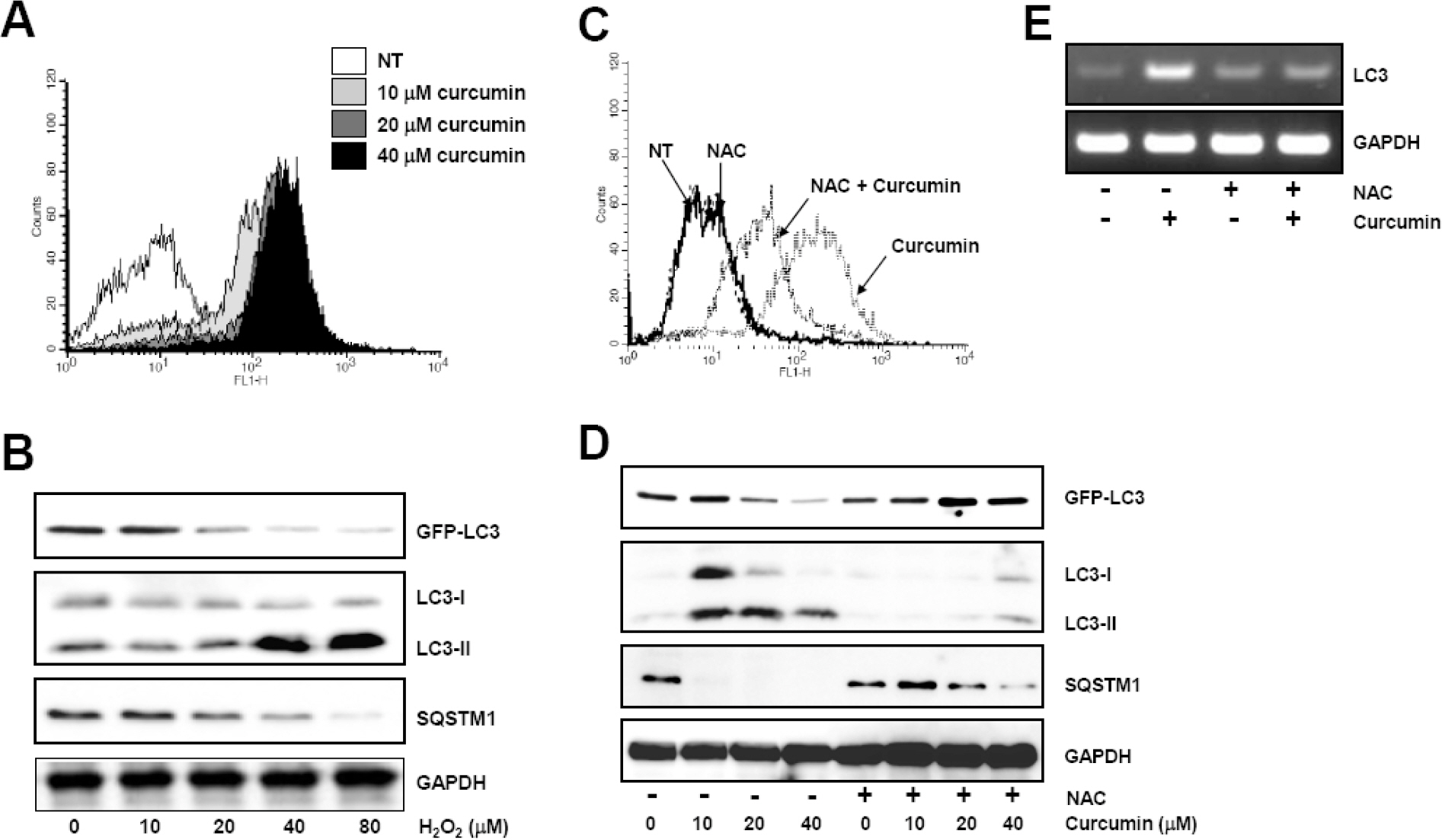
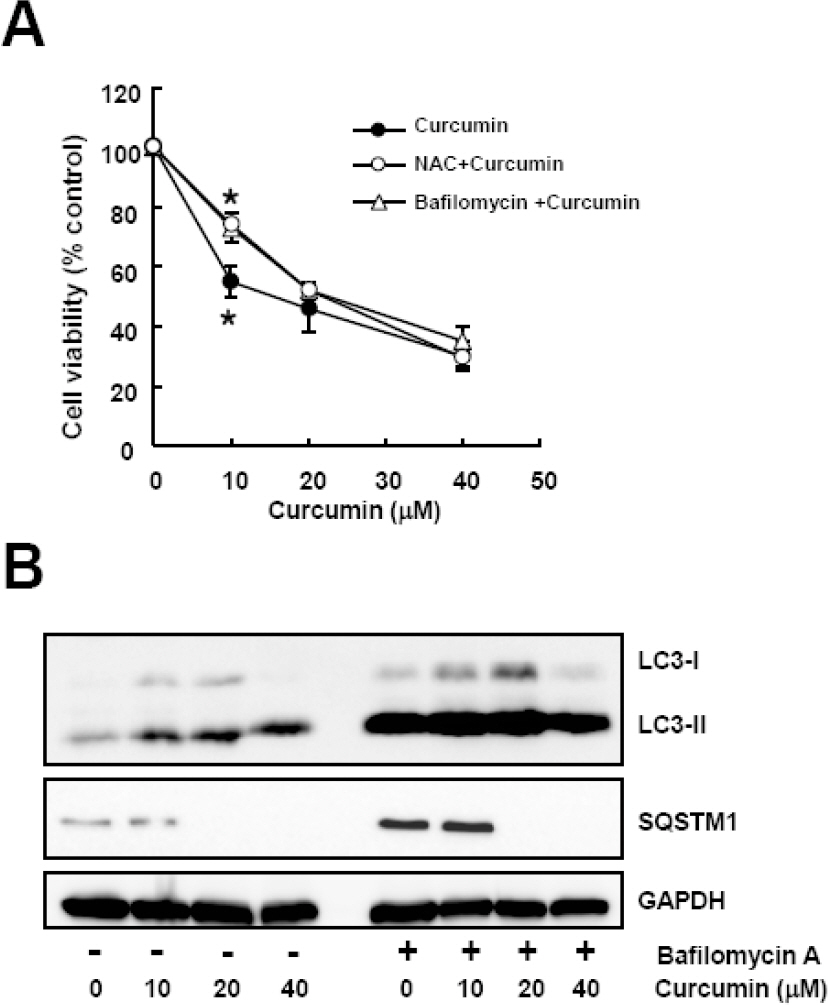
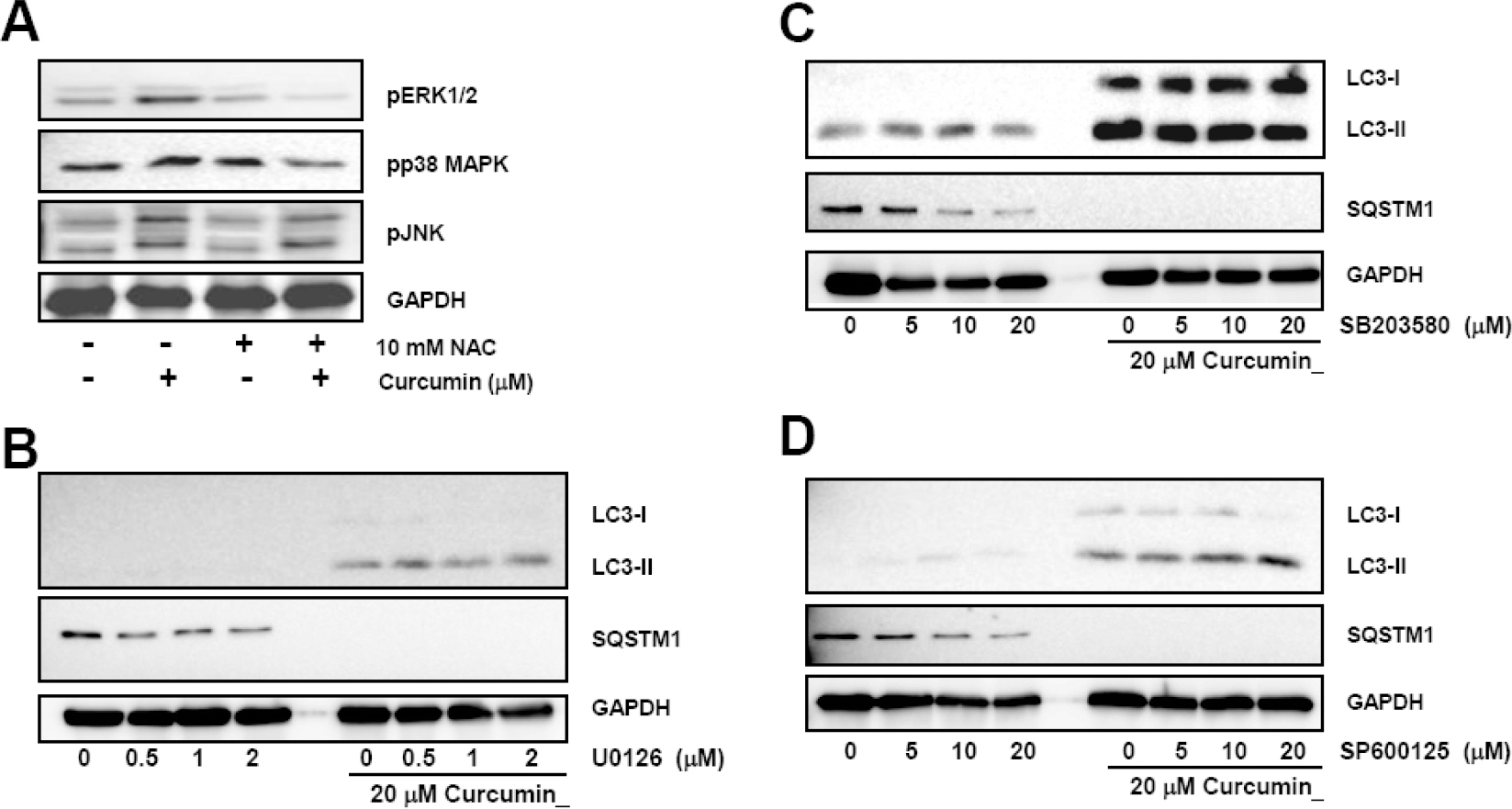
- Many anticancer agents as well as ionizing radiation have been shown to induce autophagy which is originally described as a protein recycling process and recently reported to play a crucial role in various disorders. In HCT116 human colon cancer cells, we found that curcumin, a polyphenolic phytochemical extracted from the plant Curcuma longa, markedly induced the conversion of microtubule-associated protein 1 light chain 3 (LC3)-I to LC3-II and degradation of sequestome-1 (SQSTM1) which is a marker of autophagosome degradation. Moreover, we found that curcumin caused GFP-LC3 formation puncta, a marker of autophagosome, and decrease of GFP-LC3 and SQSTM1 protein level in GFP-LC3 expressing HCT116 cells. It was further confirmed that treatment of cells with hydrogen peroxide induced increase of LC3 conversion and decrease of GFP-LC3 and SQSTM1 levels, but these changes by curcumin were almost completely blocked in the presence of antioxidant, N-acetylcystein (NAC), indicating that curcumin leads to reactive oxygen species (ROS) production, which results in autophagosome development and autolysosomal degradation. In parallel with NAC, SQSTM1 degradation was also diminished by bafilomycin A, a potent inhibitor of autophagosome-lysosome fusion, and cell viability assay was further confirmed that cucurmin-induced cell death was partially blocked by bafilomycin A as well as NAC. We also observed that NAC abolished curcumin-induced activation of extracelluar signal-regulated kinases (ERK) 1/2 and p38 mitogen-activated protein kinases (MAPK), but not Jun N-terminal kinase (JNK). However, the activation of ERK1/2 and p38 MAPK seemed to have no effect on the curcumin-induced autophagy, since both the conversion of LC3 protein and SQSTM1 degradation by curcumin was not changed in the presence of NAC. Taken together, our data suggest that curcumin induced ROS production, which resulted in autophagic activation and concomitant cell death in HCT116 human colon cancer cell. However, ROS-dependent activation of ERK1/2 and p38 MAPK, but not JNK, might not be involved in the curcumin-induced autophagy.
Keyword
MeSH Terms
-
Antineoplastic Agents
Autophagy
Cell Death
Cell Survival
Colonic Neoplasms
Curcuma
Curcumin
HCT116 Cells
Humans
Hydrogen Peroxide
Light
Macrolides
Microtubule-Associated Proteins
p38 Mitogen-Activated Protein Kinases
Phosphotransferases
Plants
Radiation, Ionizing
Reactive Oxygen Species
Recycling
Antineoplastic Agents
Curcumin
Hydrogen Peroxide
Macrolides
Microtubule-Associated Proteins
Reactive Oxygen Species
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
References
1. Huang WP, Klionsky DJ. Autophagy in yeast: a review of the molecular machinery. Cell Struct Funct. 2002; 27:409–420.
Article2. Noda T, Suzuki K, Ohsumi Y. Yeast autophagosomes: de novo formation of a membrane structure. Trends Cell Biol. 2002; 12:231–235.
Article3. Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001; 2:211–216.
Article4. Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008; 22:695–699.
Article5. Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003; 278:25009–25013.6. Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002; 11:1107–1117.
Article7. Komatsu M, Kominami E, Tanaka K. Autophagy and neuro-degeneration. Autophagy. 2006; 2:315–317.
Article8. Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004; 279:18384–18391.
Article9. Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003; 63:2103–2108.10. Kar R, Singha PK, Venkatachalam MA, Saikumar P. A novel role for MAP1 LC3 in nonautophagic cytoplasmic vacuolation death of cancer cells. Oncogene. 2009; 28:2556–2568.
Article11. Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000; 11:49–61.12. Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005; 26:1401–1410.
Article13. Mann SS, Hammarback JA. Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J Biol Chem. 1994; 269:11492–11497.
Article14. Tanida I, Tanida-Miyake E, Ueno T, Kominami E. The human homolog of Saccharomyces cerevisiae Apg7p is a protein-activating enzyme for multiple substrates including human Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem. 2001; 276:1701–1706.
Article15. Tanida I, Nishitani T, Nemoto T, Ueno T, Kominami E. Mammalian Apg12p, but not the Apg12p. Apg5p conjugate, facilitates LC3 processing. Biochem Biophys Res Commun. 2002; 296:1164–1170.16. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000; 19:5720–5728.
Article17. Bj⊘rk⊘y G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005; 171:603–614.18. Mizushima N, Yoshimori T. How to interpret LC3 immuno-blotting. Autophagy. 2007; 3:542–545.
Article19. Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999; 39:41–47.
Article20. Nagano T, Oyama Y, Kajita N, Chikahisa L, Nakata M, Okazaki E, Masuda T. New curcuminoids isolated from Zingiber cassumunar protect cells suffering from oxidative stress: a flow-cytometric study using rat thymocytes and H2O2. Jpn J Pharmacol. 1997; 75:363–370.
Article21. Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004; 10:6847–6854.22. Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008; 14:4491–4499.
Article23. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007; 595:105–125.
Article24. Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003; 24:1199–1208.
Article25. Chen Q, Wang Y, Xu K, Lu G, Ying Z, Wu L, Zhan J, Fang R, Wu Y, Zhou J. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol Rep. 2010; 23:397–403.
Article26. Lee HS, Lee MJ, Kim H, Choi SK, Kim JE, Moon HI, Park WH. Curcumin inhibits TNFalpha-induced lectin-like oxidised LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J Enzyme Inhib Med Chem. 2010; 25:720–729.27. Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, Solomides CC, Javvadi P, Koumenis C, Cengel KA, Christofidou-Solomidou M. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010; 173:590–601.
Article28. Pongrakhananon V, Nimmannit U, Luanpitpong S, Rojanasakul Y, Chanvorachote P. Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis. 2010; 15:574–585.
Article29. Kim KC, Lee CH. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; Involvement of reactive 0xygen species. Korean J Physiol Pharmacol. 2010; 14:391–397.30. Jia YL, Li J, Qin ZH, Liang ZQ. Autophagic and apoptotic mechanisms of curcumin-induced death in K562 cells. J Asian Nat Prod Res. 2009; 11:918–928.
Article31. Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007; 72:29–39.
Article32. O'Sullivan-Coyne G, O'Sullivan GC, O'Donovan TR, Piwocka K, McKenna SL. Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br J Cancer. 2009; 101:1585–1595.33. Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008; 73:1491–1501.
Article34. Ramachandiran S, Huang Q, Dong J, Lau SS, Monks TJ. Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol. 2002; 15:1635–1642.
Article35. Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000; 275:39435–39443.
Article36. Collett GP, Campbell FC. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004; 25:2183–2189.
Article37. Ramachandiran S, Huang Q, Dong J, Lau SS, Monks TJ. Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol. 2002; 15:1635–1642.
Article38. McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010; 298:C542–549.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells
- Selective Effects of Curcumin on CdSe/ZnS Quantum-dot-induced Phototoxicity Using UVA Irradiation in Normal Human Lymphocytes and Leukemia Cells
- Curcumin Induces Downregulation of E2F4 Expression and Apoptotic Cell Death in HCT116 Human Colon Cancer Cells; Involvement of Reactive Oxygen Species
- PC12 Cell Apoptosis Mediated by Reactive Oxygen Species with Various Agents is Reduced Effectively by Flavonoid Antioxidants
- Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway