J Korean Endocr Soc.
2008 Apr;23(2):129-136. 10.3803/jkes.2008.23.2.129.
Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1Department of Chemistry, Kyonggi University, Korea.
- KMID: 2063564
- DOI: http://doi.org/10.3803/jkes.2008.23.2.129
Abstract
-
BACKGROUND: An increase in oxidative stress is postulated to contribute to the development of diabetic complications and the use of antioxidant therapy could be protective against these processes. This study was performed to investigate the role of the antioxidant S-allylcysteine (SAC), a water-soluble component of aged garlic, for reducing levels of oxidative stress that occurs in diabetic rats.
METHODS
SAC (100 mg/head/day) was administered orally to streptozotocin-induced diabetic rats for eight weeks. The effects of SAC on the levels of markers of oxidative stress (malondialdehyde and glutathione) and mRNA expression of antioxidant enzymes were measured in the liver and kidney.
RESULTS
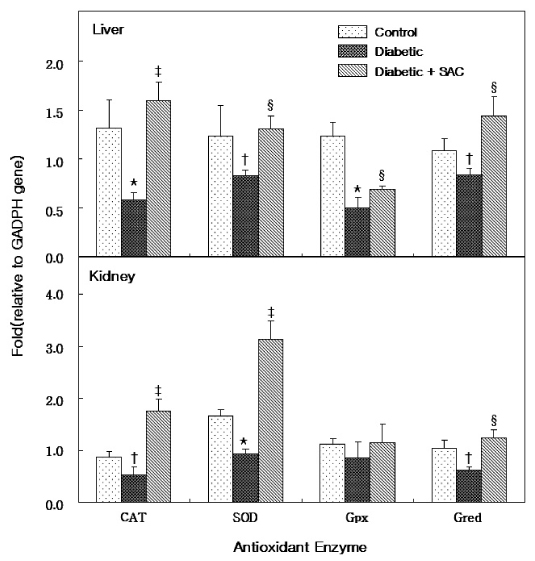
SAC-fed rats showed lower cholesterol and triacylglyceride levels than untreated diabetic rats. Malondialdehyde levels were increased in the liver and kidney of diabetic rats and SAC administration lowered the levels in both organs. Glutathione levels were lower in the liver and kidney of diabetic rats, and SAC administration restored the glutathione to a level similar in non-diabetic rats. In the liver and kidney of untreated diabetic rats, mRNA expression of catalase, superoxide dismutase and glutathione reductase were down regulated, and administration of SAC increased expression of these enzymes.
CONCLUSION
Our results have shown that administration of SAC to diabetic rats can lower blood lipid levels and alleviate oxidative stress in the diabetic tissues, suggesting that SAC might have beneficial effects in a prevention trial for diabetic complications.
MeSH Terms
Figure
Cited by 1 articles
-
Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus
Young-Min Lee, Oh-Cheon Gweon, Yeong-Ju Seo, Jieun Im, Min-Jung Kang, Myo-Jeong Kim, Jung-In Kim
Nutr Res Pract. 2009;3(2):156-161. doi: 10.4162/nrp.2009.3.2.156.
Reference
-
1. Nishikawa T, Edelstein D, Brownlee L. The missing link: a single unifying mechanism for diabetic complications. Kidney Int. 2000. 77:S26–S30.2. Kuroki T, Isshiki K, King GL. Oxidative stress: the lead or supporting actor in the pathogenesis of diabetes complications. J Am Soc Nephrol. 2003. 14:S216–S220.3. Scott JA, King GL. Oxidative stress and antioxidant treatment in diabetes. Ann N Y Acad Sci. 2004. 1031:204–213.4. Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complcations. JAMA. 2002. 288:2579–2588.5. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications. a new perspective on an old paradigm. Diabetes. 1999. 48:1–9.6. Block E. The chemistry of garlic and onions. Sci Am. 1985. 252:114–119.7. Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996. 16:111–124.8. Augusti KT. Therapeutic values of onion (Allium Cepa L) and garlic (Allium sativum L). Indian J Exp Biol. 1996. 34:634–640.9. Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003. 17:97–106.10. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994. 60:417–420.11. Moriguchi T, Saito H, Nishiyama N. Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biol Pharm Bull. 1996. 19:305–307.12. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001. 131:955S–962S.13. Yamasaki K, Hashimoto A, Kokusenya Y, Miyamoto T, Sato T. Electrochemical method for estimating the antioxidative effects of methanol extracts of crude drugs. Chem Pharm Bull(Tokyo). 1994. 42:1663–1665.14. Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J Nutr. 2001. 131:1020S–1026S.15. Mostafa MG, Mima T, Ohnishi ST, Mori K. S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice. Planta Med. 2000. 66:148–151.16. Numagami Y, Ohnishi ST. S-allylcysteine inhibits free radical production, lipid peroxidation and neuronal damage in rat brain ischemia. J Nutr. 2001. 131:1100S–1105S.17. Peng Q, Buz'Zard AR, Lau BH. Neuroprotective effect of garlic compounds in amyloid-beta peptide-induced apoptosis in vitro. Med Sci Monit. 2002. 8:BR328–BR337.18. Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, Hernádez-Pando R, Pedraza-Chaverrí J. Antioxidant S-allycysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic Biol Med. 2003. 35:317–324.19. Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006. 36:716S–725S.20. Ide N, Lau BH. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury. J Pharm Pharmacol. 1997. 49:908–911.21. Nagae S, Ushijima M, Hatono S, Imai J, Kasuga S, Matsuura H, Itakura Y, Higashi Y. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994. 60:214–217.22. Maldonado PD, Barrera D, Medina-Campos ON, Hernádez-Pando R, Ibarra-Rubio ME, Pedraza-Chaverrí J. Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Life Sci. Life Sci. 2003. 73:2543–2556.23. Ahmad MS, Ahmed N. Antiglycation properties of aged garlic extract: possible role in prevention of diabetic complications. J Nutr. 2006. 136:796S–799S.24. Ahmad MS, Pischetsrieder M, Ahmed N. Aged garlic extract and S-allylcysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 2007. 561:32–38.25. Huang CN, Horng JS, Yin MC. Antioxidative and antiglycative effects of six organo sulfur compounds in low-density lipoprotein and plasma. J Agric Food Chem. 2004. 52:3674–3678.26. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.27. Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985. 113:548–555.28. Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002. 1:4–9.29. Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr. 2001. 131:977S–979S.30. Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001. 31:1010S–1015S.31. Nagae S, Ushijima M, Hatono S, Imai J, Kasuga S, Matsuura H, Itakura Y, Higashi Y. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994. 60:214–217.32. Hsu CC, Yen HF, Yin MC, Tsai CM, Hsieh CH. Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr. 2004. 134:3245–3249.33. Baştar I, Seckin S, Uysal M, Aykaç-Toker G. Effect of streptozotocin on glutathione and lipid peroxide levels in various tissues of rats. Res Commun Mol Pathol Pharmacol. 1998. 102:265–272.34. Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Increased oxidative stress in rat liver and pancrease during progression of streptozotocin-induced diabetes. Clin Sci. 1998. 94:623–632.35. Kedziora-Kornatowska K, Luciak M. Effect of aminoguanidine on lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney. Biochem Mol Biol Int. 1998. 46:577–583.36. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress and antioxidants. J Biochem Mol Toxicol. 2003. 17:24–38.37. Inoue M, Saito Y, Hirato E, Morino Y, Nagase S. Regulation of redox status of plasma proteins by metabolism and transport of glutathione and related compounds. J Protein Chem. 1987. 36:169–173.38. Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003. 243:147–152.39. Lal MA, Körner A, Matsuo Y, Zelenin S, Cheng SX, Jaremko G, DiBona GF, Eklöf AC, Aperia A. Combined antioxidant and COMT inhibitor treatment reverses renal abnormalities in diabetic rats. Diabetes. 2000. 49:1381–1389.40. Agardh CD, Israelsson B, Thuesen-Olesen B, Agardh E. Application of quantitative competitive polymerase chain reaction for measurements of mRNA from antioxidative enzymes in the diabetic rat retina and kidney. Metabolism. 2002. 51:1279–1284.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- Amelioration of diabetic microalbuminuria and lipid peroxidation by captopril
- Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats
- The Effect of Pinitol on Cataractogenesis and Anti-oxidative effect in Streptozotocin Induced Diabetic Rats
- The oxidative modification of hepatic intracellular proteins in the streptozotocin-induced diabetic rats