Anat Cell Biol.
2012 Jun;45(2):103-113. 10.5115/acb.2012.45.2.103.
Protective efficacy of an Ecklonia cava extract used to treat transient focal ischemia of the rat brain
- Affiliations
-
- 1Department of Anatomy, Myunggok Research Institute, Konyang University College of Medicine, Daejeon, Korea. jjzzy@konyang.ac.kr
- 2Department of Pathology, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2005873
- DOI: http://doi.org/10.5115/acb.2012.45.2.103
Abstract
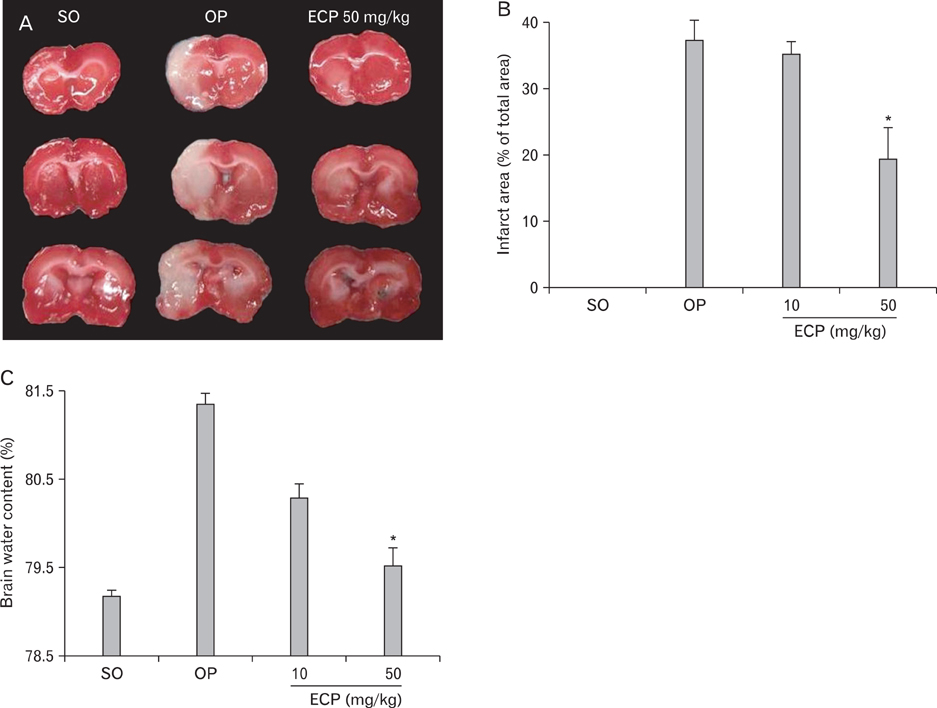
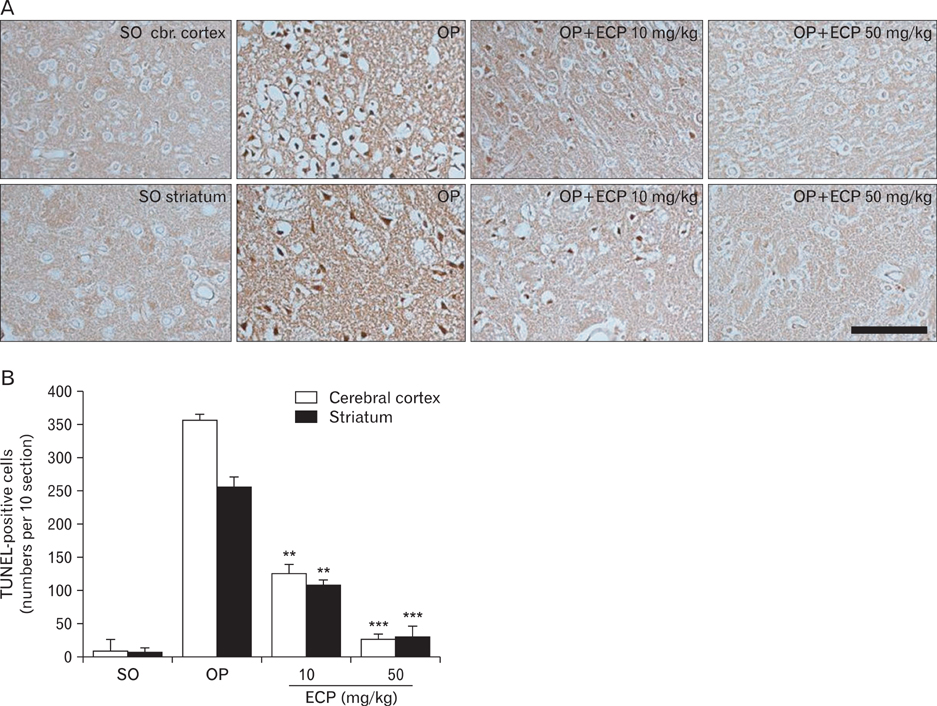
- Phlorotannins (marine algal polyphenols) have been reported to exhibit beneficial biological activities, serving as both antioxidants and anti-inflammatory agents. Among marine algae, Ecklonia cava, a member of the Laminariaceae, is a very popular food regarded as healthy in Korea and Japan. Recently, benefits afforded by phlorotannins in the treatment of various clinical conditions have been reported, but any therapeutic effects of such materials in the treatment of neurodegenerative diseases such as stroke remain unclear. Also, the mechanisms of action of the algal components remain poorly understood. In the present in vivo study, administration of Ecklonia cava polyphenols (ECP) at 10 mg/kg and 50 mg/kg intraperitoneally (i.p.) significantly decreased infarct size and the extent of brain edema in the rat after induction of transient focal ischemia via middle cerebral artery occlusion (MCAO). Further, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay revealed dose-dependent blockage of neuronal apoptosis upon intravenous ECP treatment. Neurobehavioral tests performed over the 6 days after MCAO revealed a reduction in neurological motor performance in control animals, but administration of ECP (50 mg/kg i.p.) prevented this decline. In vitro, a significant neuroprotective effect of ECP was evident when cell viability was assayed after induction of H2O2-mediated oxidative stress, upon retinoic acid treatment, in the differentiated neuroblastoma cell line SH-SY5Y. Interestingly, ECP blocked the rise in cytosolic calcium, in a dose-dependent manner, in differentiated SH-SY5Y cells exposed to H2O2. Together, the results suggest that ECP exerts neuroprotective effects in the focally ischemic brain by reducing Ca(2+)-mediated neurotoxicity.
MeSH Terms
-
Animals
Anti-Inflammatory Agents
Antioxidants
Apoptosis
Brain
Brain Edema
Calcium
Cell Line
Cell Survival
Cytosol
Infarction, Middle Cerebral Artery
Ischemia
Japan
Korea
Neuroblastoma
Neurodegenerative Diseases
Neurons
Neuroprotective Agents
Oxidative Stress
Polyphenols
Rats
Stroke
Tretinoin
Anti-Inflammatory Agents
Antioxidants
Calcium
Neuroprotective Agents
Polyphenols
Tretinoin
Figure
Reference
-
1. Schlaepfer WW, Bunge RP. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J Cell Biol. 1973. 59(2 Pt 1):456–470.2. Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979. 206:700–702.3. Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985. 58:293–297.4. Arundine M, Aarts M, Lau A, Tymianski M. Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci. 2004. 24:8106–8123.5. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010. 47:122–129.6. Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998. 56:317–333.7. Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995. 22:375–383.8. Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997. 2:152–159.9. Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998. 5:401–414.10. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001. 74:418–425.11. Acheson RM, Williams DR. Does consumption of fruit and vegetables protect against stroke? Lancet. 1983. 1:1191–1193.12. Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999. 282:1233–1239.13. Kang HS, Chung HY, Jung JH, Son BW, Choi JS. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem Pharm Bull (Tokyo). 2003. 51:1012–1014.14. Zou Y, Qian ZJ, Li Y, Kim MM, Lee SH, Kim SK. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agric Food Chem. 2008. 56:7001–7009.15. Kang KA, Lee KH, Park JW, Lee NH, Na HK, Surh YJ, You HJ, Chung MH, Hyun JW. Triphlorethol-A induces heme oxygenase-1 via activation of ERK and NF-E2 related factor 2 transcription factor. FEBS Lett. 2007. 581:2000–2008.16. Okada Y, Ishimaru A, Suzuki R, Okuyama T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: potential for the effective treatment of diabetic complications. J Nat Prod. 2004. 67:103–105.17. Yoon NY, Chung HY, Kim HR, Choi JS. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish Sci. 2008. 74:200–207.18. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995. 26:627–634.19. Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996. 27:1616–1622.20. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.21. Schwab M, Bauer R, Zwiener U. The distribution of normal brain water content in Wistar rats and its increase due to ischemia. Brain Res. 1997. 749:82–87.22. Nguyen TT, Cho SO, Ban JY, Kim JY, Ju HS, Koh SB, Song KS, Seong YH. Neuroprotective effect of Sanguisorbae Radix against oxidative stress-induced brain damage: in vitro and in vivo. Biol Pharm Bull. 2008. 31:2028–2035.23. Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005. 31:135–147.24. Wexler BC. Metabolic changes in response to acute cerebral ischemia following bilateral carotid artery ligation in arteriosclerotic versus nonarteriosclerotic rats. Stroke. 1970. 1:112–121.25. Martins E, Inamura K, Themner K, Malmqvist KG, Siesjö BK. Accumulation of calcium and loss of potassium in the hippocampus following transient cerebral ischemia: a proton microprobe study. J Cereb Blood Flow Metab. 1988. 8:531–538.26. Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988. 11:465–469.27. Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia. 2005. 50:287–298.28. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010. 1802:80–91.29. Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol Res. 2000. 33:55–64.30. Dajas F, Rivera-Megret F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, Echeverry C, Lafon L, Heizen H, Ferreira M, Morquio A. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003. 36:1613–1620.31. Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008. 476:107–112.32. Pahlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984. 14:135–144.33. Adem A, Mattsson ME, Nordberg A, Påhlman S. Muscarinic receptors in human SH-SY5Y neuroblastoma cell line: regulation by phorbol ester and retinoic acid-induced differentiation. Brain Res. 1987. 430:235–242.34. Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ. Eukaryotic Signal Transduction Group. Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron. 1993. 11:321–331.35. Perez-Polo JR, Werbach-Perez K, Tiffany-Castiglioni E. A human clonal cell line model of differentiating neurons. Dev Biol. 1979. 71:341–355.36. Ammer H, Schulz R. Retinoic acid-induced differentiation of human neuroblastoma SH-SY5Y cells is associated with changes in the abundance of G proteins. J Neurochem. 1994. 62:1310–1318.37. Klatzo I. Presidental address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967. 26:1–14.38. Katzman R, Clasen R, Klatzo I, Meyer JS, Pappius HM, Waltz AG. Report of Joint Committee for Stroke Resources. IV. Brain edema in stroke. Stroke. 1977. 8:512–540.39. Albers GW. Diffusion-weighted MRI for evaluation of acute stroke. Neurology. 1998. 51:3 Suppl 3. S47–S49.40. Siesjö BK. Cerebral circulation and metabolism. J Neurosurg. 1984. 60:883–908.41. Vexler ZS, Roberts TP, Bollen AW, Derugin N, Arieff AI. Transient cerebral ischemia. Association of apoptosis induction with hypoperfusion. J Clin Invest. 1997. 99:1453–1459.42. Mellergård P, Bengtsson F, Smith ML, Riesenfeld V, Siesjö BK. Time course of early brain edema following reversible forebrain ischemia in rats. Stroke. 1989. 20:1565–1570.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effect of Focal Ischemic Preconditioning in Transient Focal Ischemia Animal Model
- Protective Effects of the Green Tea Polyphenol(-)-epigallocatechin Gallate Against Ischemia Reperfusion Injury Induced by Middle Cerebral Artery Occlusion in Rats
- Selective Gray Matter Infarction in the Basal Ganglia Associated With Transient Ischemic Attack
- Application of Ecklonia cava Kjellman by-product as a feed additive: enhancing weight gain, immunity and protection from Salmonella infection in chickens
- Comparison of the Cerebral Protective Effects of Thiopental, Propofol and Dantrolene on Focal Cerebral Ischemia Induced by Temporary Middle Cerebral Artery Occlusion in the Rat Under the Monitoring of Compressed Spectral Array