Korean J Physiol Pharmacol.
2009 Oct;13(5):349-356. 10.4196/kjpp.2009.13.5.349.
Ape1/Ref-1 Stimulates GDNF/GFR alpha1-mediated Downstream Signaling and Neuroblastoma Proliferation
- Affiliations
-
- 1Department of Pharmacology, DNA Repair Center, Chosun University School of Medicine, Gwangju 501-759, Korea. 9766m@hanmail.net
- 2Department of Rehabilitation, Chosun University Hospital, Gwangju 501-717, Korea.
- 3Department of Orthopaedic Surgery, Chosun University Hospital, Gwangju 501-717, Korea.
- KMID: 1982567
- DOI: http://doi.org/10.4196/kjpp.2009.13.5.349
Abstract
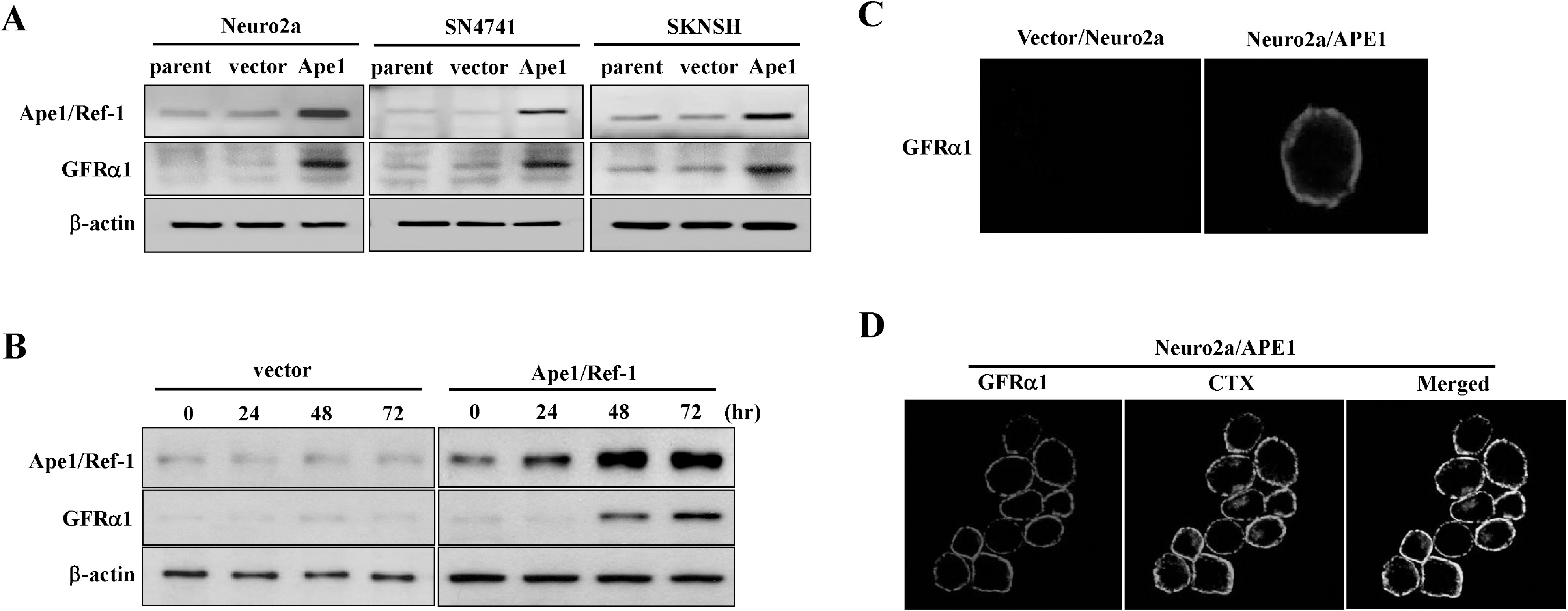
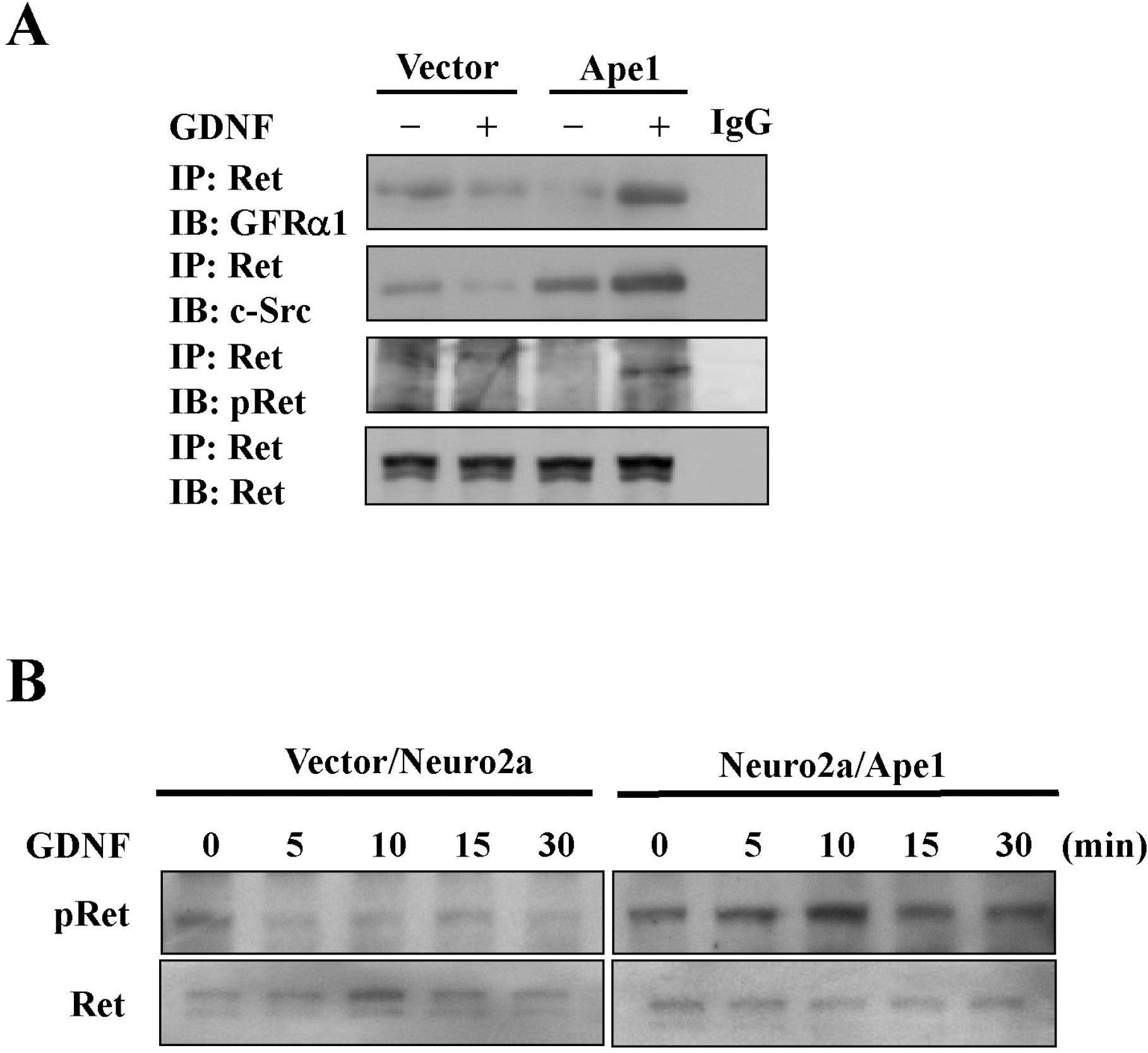
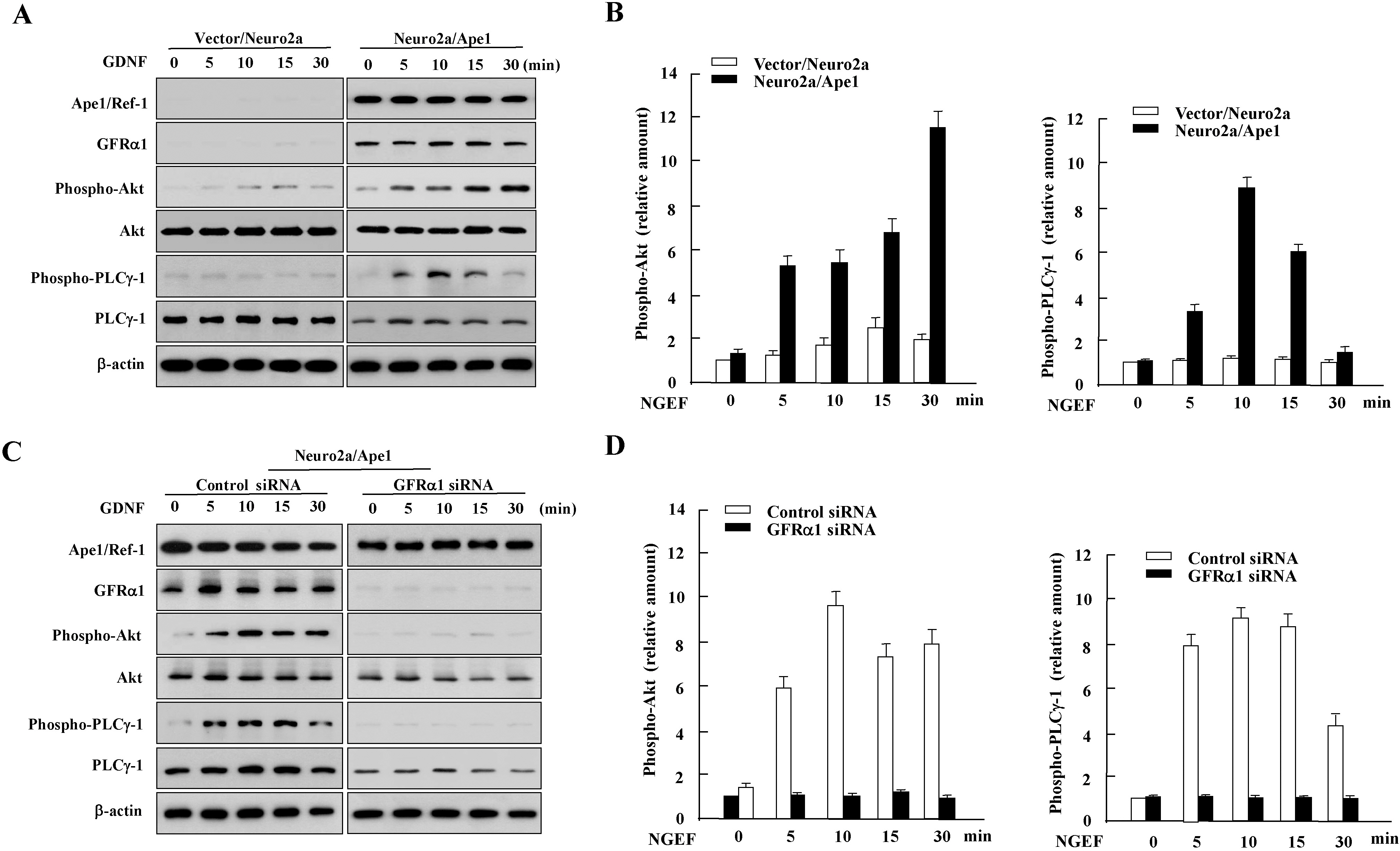
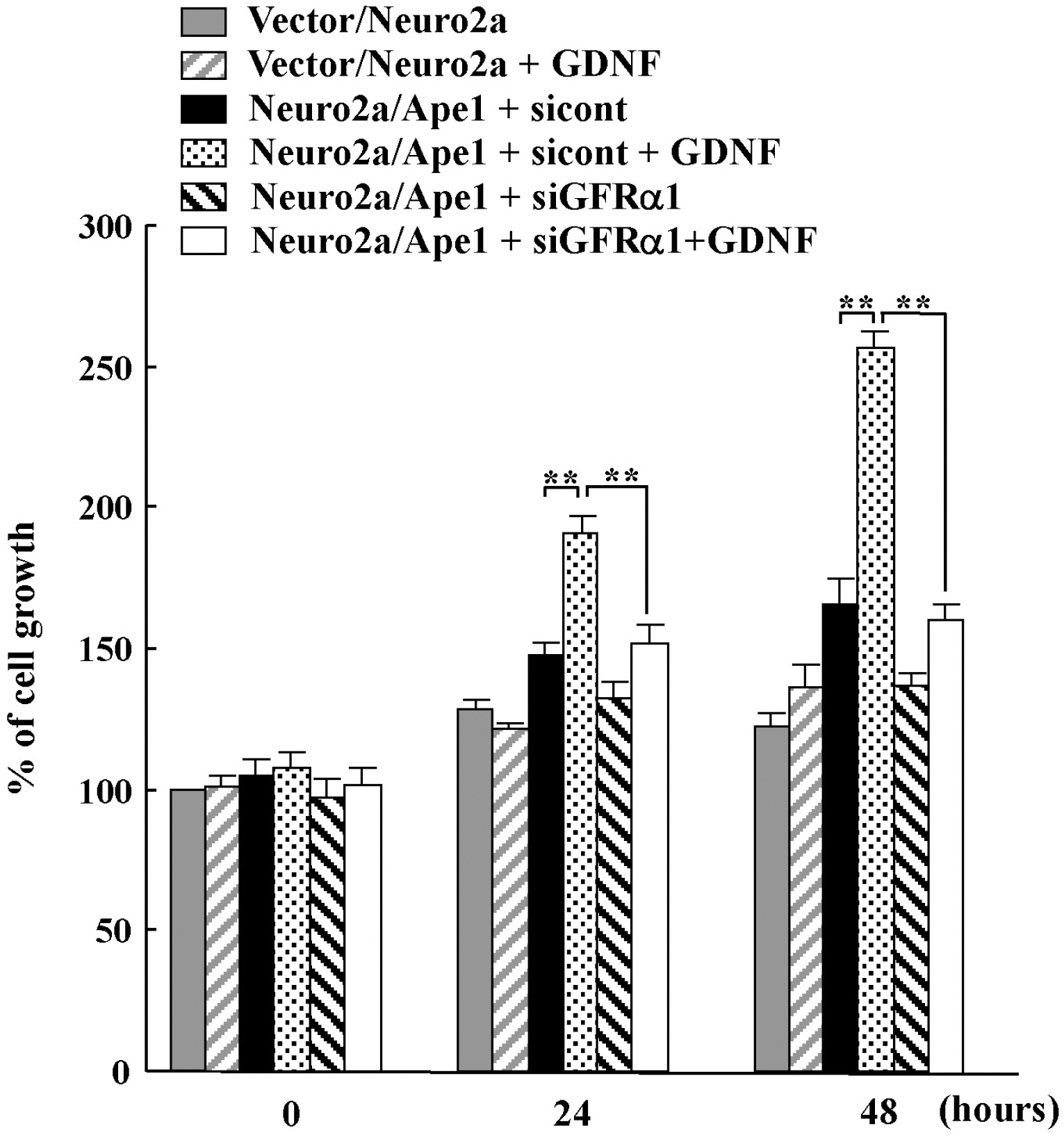
- We previously reported that glial cell line-derived neurotropic factor (GDNF) receptor alpha1 (GFR alpha1) is a direct target of apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1). In the present study, we further analyzed the physiological roles of Ape1/Ref-1-induced GFRalpha1 expression in Neuro2a mouse neuroblastoma cells. Ape1/Ref-1 expression caused the clustering of GFR alpha1 immunoreactivity in lipid rafts in response to GDNF. We also found that Ret, a downstream target of GFRalpha1, was functionally activated by GDNF in Ape1/Ref-1-expressing cells. Moreover, GDNF promoted the proliferation of Ape1/Ref-1-expressing Neuro2a cells. Furthermore, GFR alpha1-specific RNA experiments demonstrated that the downregulation of GFR alpha1 by siRNA in Ape1/Ref-1-expressing cells impaired the ability of GDNF to phosphorylate Akt and PLC gamma-1 and to stimulate cellular proliferation. These results show an association between Ape1/Ref-1 and GDNF/GFR alpha signaling, and suggest a potential molecular mechanism for the involvement of Ape1/Ref-1 in neuronal proliferation.
Keyword
MeSH Terms
Figure
Reference
-
References
Airaksinen MS., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 3:383–394. 2002.
ArticleAiraksinen MS., Titievsky A., Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 13:313–325. 1999.
ArticleAnderson RG. The caveolae membrane system. Annu Rev Biochem. 67:199–225. 1998.
ArticleBesset V., Scott RP., Ibanez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 275:39159–39166. 2000.
ArticleBobola MS., Finn LS., Ellenbogen RG., Geyer JR., Berger MS., Braga JM., Meade EH., Gross ME., Silber JR. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 11:7405–7414. 2005.
ArticleBorrello MG., Alberti L., Arighi E., Bongarzone I., Battistini C., Bardelli A., Pasini B., Piutti C., Rizzetti MG., Mondellini P., Radice MT., Pierotti MA. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol Cell Biol. 16:2151–2163. 1996.
ArticleCacalano G., Farinas I., Wang LC., Hagler K., Forgie A., Moore M., Armanini M., Phillips H., Ryan AM., Reichardt LF., Hynes M., Davies A., Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 21:53–62. 1998.Chattopadhyay R., Das S., Maiti AK., Boldogh I., Xie J., Hazra TK., Kohno K., Mitra S., Bhakat KK. Regulatory role of human AP-endonuclease (APE1/Ref-1) in YB-1-mediated activation of the multidrug resistance gene MDR1. Mol Cell Biol. 28:7066–7080. 2008.Chiarini LB., Freitas FG., Petrs-Silva H., Linden R. Evidence that the bifunctional redox factor / AP endonuclease Ref-1 is an anti-apoptotic protein associated with differentiation in the developing retina. Cell Death Differ. 7:272–281. 2000.
ArticleCoulpier M., Anders J., Ibanez CF. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem. 277:1991–1999. 2002.Durbec P., Marcos-Gutierrez CV., Kilkenny C., Grigoriou M., Wartiowaara K., Suvanto P., Smith D., Ponder B., Costantini F., Saarma M. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 381:789–793. 1996.
ArticleEdwards M., Rassin DK., Izumi T., Mitra S., Perez-Polo JR. APE/Ref-1 responses to oxidative stress in aged rats. J Neurosci Res. 54:635–638. 1998.
ArticleErnsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 333:353–371. 2008.
ArticleFantini D., Vascotto C., Deganuto M., Bivi N., Gustincich S., Marcon G., Quadrifoglio F., Damante G., Bhakat KK., Mitra S., Tell G. APE1/Ref-1 regulates PTEN expression mediated by Egr-1. Free Radic Res. 42:20–29. 2008.
ArticleFishel ML., He Y., Reed AM., Chin-Sinex H., Hutchins GD., Mendonca MS., Kelley MR. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst). 7:177–186. 2008.
ArticleFung H., Demple B. A vital role for Ape1/Ref1 protein in repairing spontaneous DNA damage in human cells. Mol Cell. 17:463–470. 2005.
ArticleFung H., Liu P., Demple B. ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis. Mol Cell Biol. 27:8834–8847. 2007.
ArticleGash DM., Zhang Z., Ovadia A., Cass WA., Yi A., Simmerman L., Russell D., Martin D., Lapchak PA., Collins F., Hoffer BJ., Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 380:252–255. 1996.
ArticleHong M., Mukhida K., Mendez I. GDNF therapy for Parkinson's disease. Expert Rev Neurother. 8:1125–1139. 2008.
ArticleIwamori M., Shimomura J., Nagai Y. Specific binding of cholera toxin to rat erythrocytes revealed by analysis with a fluorescence-activated cell sorter. J Biochem. 97:729–735. 1985.Jiang Y., Guo C., Vasko MR., Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 68:6425–6434. 2008.
ArticleJing S., Wen D., Yu Y., Holst PL., Luo Y., Fang M., Tamir R., Antonio L., Hu Z., Cupples R., Louis JC., Hu S., Altrock BW., Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 85:1113–1124. 1996.Kim MH., Kim HB., Acharya S., Sohn HM., Jun JY., Chang IY., You HJ. Ape1/Ref-1 induces glial cell-derived neurotropic factor (GDNF) responsiveness by upregulating GDNF receptor alpha1 expression. Mol Cell Biol. 29:2264–2277. 2009.Kirik D., Georgievska B., Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 7:105–110. 2004.
ArticleKisby GE., Milne J., Sweatt C. Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue. Neuroreport. 8:1337–1340. 1997.
ArticleKlein SM., Behrstock S., McHugh J., Hoffmann K., Wallace K., Suzuki M., Aebischer P., Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 16:509–521. 2005.
ArticleLarsen E., Meza TJ., Kleppa L., Klungland A. Organ and cell specificity of base excision repair mutants in mice. Mutat Res. 614:56–68. 2007.
ArticleLewen A., Sugawara T., Gasche Y., Fujimura M., Chan PH. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol Dis. 8:380–390. 2001.Lin LF., Doherty DH., Lile JD., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 260:1130–1132. 1993.
ArticleLiu H., Colavitti R., Rovira II., Finkel T. Redox-dependent transcriptional regulation. Circ Res. 97:967–974. 2005.
ArticleMcNeill DR., Lam W., DeWeese TL., Cheng YC., Wilson DM 3rd. Impairment of APE1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res. 7:897–906. 2009.
ArticleMcNeill DR., Wilson DM 3rd. A dominant-negative form of the major human abasic endonuclease enhances cellular sensitivity to laboratory and clinical DNA-damaging agents. Mol Cancer Res. 5:61–70. 2007.
ArticleMoore MW., Klein RD., Farinas I., Sauer H., Armanini M., Phillips H., Reichardt LF., Ryan AM., Carver-Moore K., Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 382:76–79. 1996.
ArticleOlkowski ZL. Mutant AP endonuclease in patients with amyotrophic lateral sclerosis. Neuroreport. 9:239–242. 1998.
ArticleOno Y., Matsumoto K., Furuta T., Ohmoto T., Akiyama K., Seki S. Relationship between expression of a major apurinic/apyrimidinic endonuclease (APEX nuclease) and susceptibility to genotoxic agents in human glioma cell lines. J Neurooncol. 25:183–192. 1995.
ArticleParatcha G., Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 31:384–391. 2008.Paratcha G., Ledda F., Baars L., Coulpier M., Besset V., Anders J., Scott R., Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 29:171–184. 2001.Pichel JG., Shen L., Sheng HZ., Granholm AC., Drago J., Grinberg A., Lee EJ., Huang SP., Saarma M., Hoffer BJ., Sariola H., Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 382:73–76. 1996.
ArticlePong K., Xu RY., Baron WF., Louis JC., Beck KD. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. J Neurochem. 71:1912–1919. 1998.
ArticleRass U., Ahel I., West SC. Defective DNA repair and neurodegenerative disease. Cell. 130:991–1004. 2007.
ArticleSaarma M., Sariola H. Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF). Microsc Res Tech. 45:292–302. 1999.
ArticleSakurai M., Nagata T., Abe K., Horinouchi T., Itoyama Y., Tabayashi K. Oxidative damage and reduction of redox factor-1 expression after transient spinal cord ischemia in rabbits. J Vasc Surg. 37:446–452. 2003.
ArticleSanchez MP., Silos-Santiago I., Frisen J., He B., Lira SA., Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 382:70–73. 1996.
ArticleSariola H., Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 116:3855–3862. 2003.
ArticleTan Z., Sun N., Schreiber SS. Immunohistochemical localization of redox factor-1 (Ref-1) in Alzheimer's hippocampus. Neuroreport. 9:2749–2752. 1998.
ArticleTansey MG., Baloh RH., Milbrandt J., Johnson EM. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 25:611–623. 2000.Tomac A., Lindqvist E., Lin LF., Ogren SO., Young D., Hoffer BJ., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 373:335–339. 1995.
ArticleTreanor JJ., Goodman L., de Sauvage F., Stone DM., Poulsen KT., Beck CD., Gray C., Armanini MP., Pollock RA., Hefti F., Phillips HS., Goddard A., Moore MW., Buj-Bello A., Davies AM., Asai N., Takahashi M., Vandlen R., Henderson CE., Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 382:80–83. 1996.
ArticleTsui-Pierchala BA., Encinas M., Milbrandt J., Johnson EM. Lipid rafts in neuronal signaling and function. Trends Neurosci. 25:412–417. 2002.
ArticleVasko MR., Guo C., Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair (Amst). 4:367–379. 2005.
ArticleWalton M., Lawlor P., Sirimanne E., Williams C., Gluckman P., Dragunow M. Loss of Ref-1 protein expression precedes DNA fragmentation in apoptotic neurons. Brain Res Mol Brain Res. 44:167–170. 1997.
ArticleWang D., Luo M., Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 3:679–686. 2004.Wilson TM., Rivkees SA., Deutsch WA., Kelley MR. Differential expression of the apurinic / apyrimidinic endonuclease (APE/ref-1) multifunctional DNA base excision repair gene during fetal development and in adult rat brain and testis. Mutat Res. 362:237–248. 1996.Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. Embo J. 11:653–665. 1992.
ArticleXanthoudakis S., Smeyne RJ., Wallace JD., Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci USA. 93:8919–8923. 1996.
ArticleXiang DB., Chen ZT., Wang D., Li MX., Xie JY., Zhang YS., Qing Y., Li ZP., Xie J. Chimeric adenoviral vector Ad5/F35-mediated APE1 siRNA enhances sensitivity of human colorectal cancer cells to radiotherapy in vitro and in vivo. Cancer Gene Ther. 15:625–635. 2008.
ArticleYang ZZ., Chen XH., Wang D. Experimental study enhancing the chemosensitivity of multiple myeloma to melphalan by using a tissue-specific APE1-silencing RNA expression vector. Clin Lymphoma Myeloma. 7:296–304. 2007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aspirin-induced acetylation of APE1/Ref-1 enhances RAGE binding and promotes apoptosis in ovarian cancer cells
- Altered Secretory Activity of APE1/Ref-1 D148E Variants Identified in Human Patients With Bladder Cancer
- Role of APE1/Ref-1 in hydrogen peroxide-induced apoptosis in human renal HK-2 cells
- Alterations in Adipose Tissue and Adipokines in Heterozygous APE1/Ref-1 Deficient Mice
- Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein