Lab Anim Res.
2011 Sep;27(3):259-263. 10.5625/lar.2011.27.3.259.
Esculetin inhibits N-methyl-D-aspartate neurotoxicity via glutathione preservation in primary cortical cultures
- Affiliations
-
- 1College of Veterinary Medicine and Institute of Animal Medicine, Kangwon National University, Chuncheon, Korea. mbwie@kangwon.ac.kr
- 2Neuropsychopharmacology and Toxicology Program, College of Pharmacy, Kangwon National University, Chuncheon, Korea.
- 3Department of Laboratory Animal Science, Korea Bio Polytechnic College, Nonsan, Korea.
- 4Department of Veterinary Medicine, Cheju National University, Jeju, Korea.
- KMID: 1850055
- DOI: http://doi.org/10.5625/lar.2011.27.3.259
Abstract
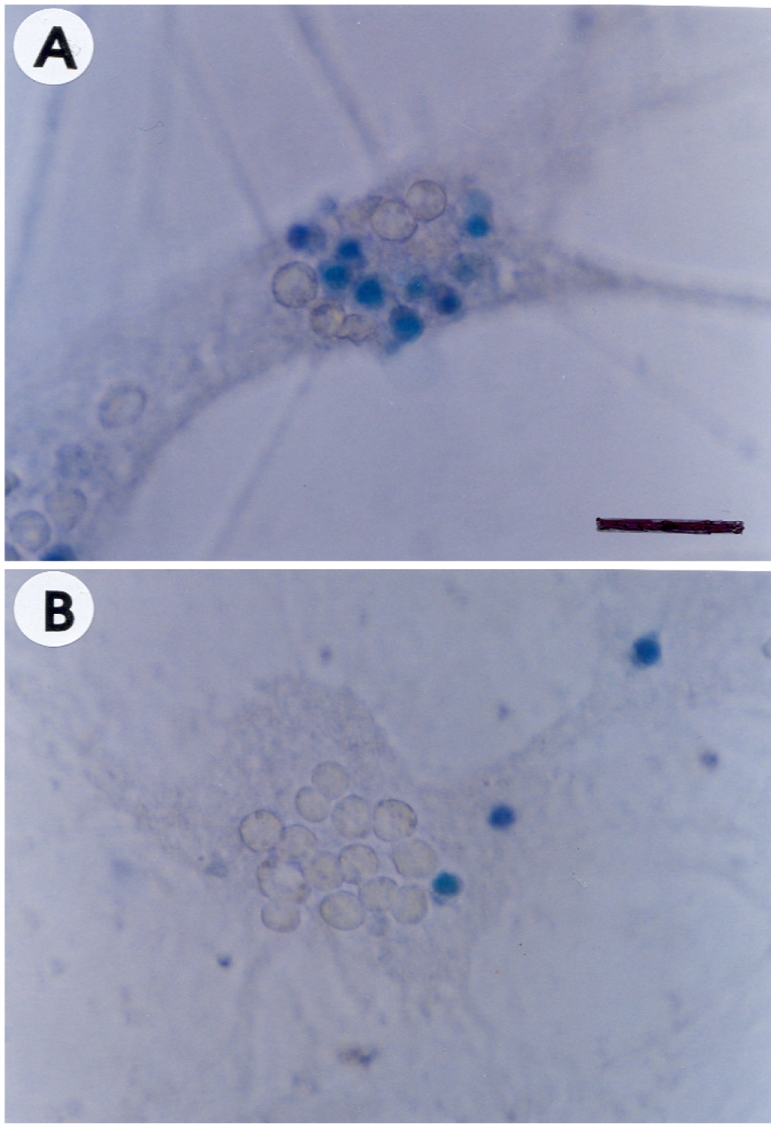
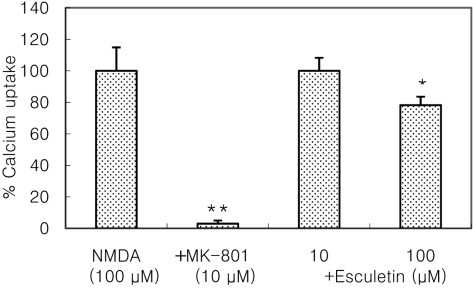
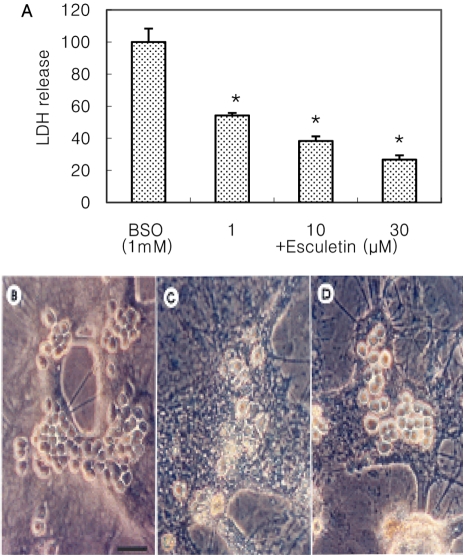
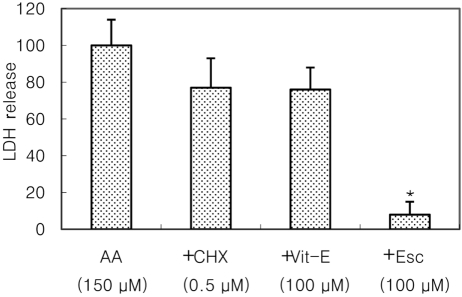
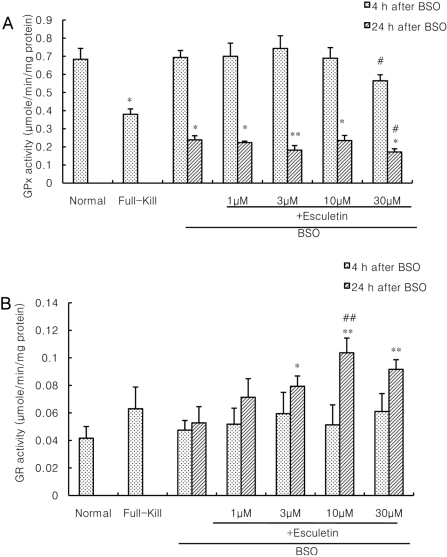
- Recently, loss of endogenous glutathione during N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxic injury, and the resultant overproduction of reactive oxygen species (ROS) through an arachidonic acid cascade process in brain, have been implicated in neuronal damage in various neurodegenerative diseases. Glutathione depletion induced by L-buthionine-(S,R)-sulfoximine (BSO), an inhibitor of glutathione synthesis, is known to cause arachidonic acid-mediated excitotoxicity in primary mixed cortical cultures. The aim of this study was to investigate whether esculetin (6,7-dihydroxycoumarin), an inhibitor of lipoxygenase, protects against neurotoxicity induced by NMDA or BSO. We observed that neurotoxicity induced by NMDA but not kainic acid was attenuated by esculetin. At the same concentration (100 microM), esculetin attenuated the 45Ca2+ uptake elevation induced by NMDA. Free radical-mediated neuronal injury induced by H2O2 and xanthine/xanthine oxidase was concentration-dependently blocked by esculetin. Esculetin (1-30 microM) dose-dependently inhibited BSO-induced neuronal injury. In addition, arachidonate-induced neurotoxicity was completely blocked by esculetin. BSO also reduced glutathione peroxidase (GPx) activity, but did not change glutathione reductase (GR) activity 24 h after treatment. Esculetin dose-dependently increased GR activity, but did not alter GPx activity. These findings suggest that esculetin can contribute to the rescue of neuronal cells from NMDA neurotoxicity and that this protective effect occurs partly through NMDA receptor modulation and the sparing of glutathione depletion.
Keyword
MeSH Terms
-
Arachidonic Acid
Brain
Glutathione
Glutathione Reductase
Kainic Acid
Lipoxygenase
N-Methylaspartate
Neurodegenerative Diseases
Neurons
Oxidoreductases
Peroxidase
Reactive Oxygen Species
Umbelliferones
Arachidonic Acid
Glutathione
Glutathione Reductase
Kainic Acid
Lipoxygenase
N-Methylaspartate
Oxidoreductases
Peroxidase
Reactive Oxygen Species
Umbelliferones
Figure
Reference
-
1. Dumuis A, Sebben M, Haynes L, Pin JP, Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988; 336(6194):68–70. PMID: 2847054.
Article2. Almeida A, Heales SJ, Bolaños JP, Medina JM. Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res. 1998; 790(1-2):209–216. PMID: 9593899.
Article3. Taylor AL, Hewett SJ. Potassium-evoked glutamate release liberates arachidonic acid from cortical neurons. J Biol Chem. 2002; 277(46):43881–43887. PMID: 12235140.
Article4. Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990; 11(7):290–296. PMID: 2167544.
Article5. Rodriguez-Alvarez J, Blanco I, Patel AJ. Modulation of N-methyl-D-aspartate receptor mediated arachidonic acid release by endogenous nitric oxide in cultured neurons. Neurosci Lett. 1996; 218(2):95–98. PMID: 8945736.6. Ge QF, Wie EQ, Zhang WP, Hu X, Huang XJ, Zhang L, Song Y, Ma ZQ, Chen Z, Luo JH. Activation of 5-lipoxygenase after oxygen-glucose deprivation is partly mediated via NMDA receptor in rat cortical neurons. J Neurochem. 2006; 97(4):992–1004. PMID: 16606359.
Article7. Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory antioxidant activities. Curr Pharm Des. 2004; 10(30):3813–3833. PMID: 15579073.8. Bridges RJ, Koh JY, Hatalski CG, Cotman CW. Increased excitotoxic vulnerability of cortical cultures with reduced levels of glutathione. Eur J Pharmacol. 1991; 192(1):199–200. PMID: 1674918.
Article9. Regan RF, Guo YP. Potentiation of excitotoxic injury by high concentrations of extracellular reduced glutathione. Neuroscience. 1999; 91(2):463–470. PMID: 10366003.
Article10. Regan RF, Guo Y. Extracellular reduced glutathione increases neuronal vulnerability to combined chemical hypoxia and glucose deprivation. Brain Res. 1999; 817(1-2):145–150. PMID: 9889354.
Article11. Wie MB, Won MH, Lee KH, Shin JH, Lee JC, Suh HW, Song DK, Kim YH. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci Lett. 1997; 225(2):93–96. PMID: 9147382.
Article12. Kim SR, Park MJ, Lee MK, Sung SH, Park EJ, Kim J, Kim SY, Oh TH, Markelonis GJ, Kim YC. Flavonoids of Inula Britannica protect cultured cortical cells from necrotic cell death induced by glutamate. Free Radic Biol Med. 2002; 32(7):596–604. PMID: 11909694.
Article13. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976; 71(4):952–958. PMID: 971321.
Article14. Eklöw L, Moldéus P, Orrenius S. Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocyte and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem. 1984; 138(3):459–463. PMID: 6692829.15. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193(1):265–275. PMID: 14907713.
Article16. Kim HC, Jhoo WK, Bing G, Shin EJ, Wie MB, Kim WK, Ko KH. Phenidone prevents kainate-induced neurotoxicity via antioxidant mechanisms. Brain Res. 2000; 874(1):15–23. PMID: 10936219.
Article17. Taylor AL, Bonventre JV, Uliasz TF, Hewett JA, Hewett SJ. Cytosolic phospholipase A2 alpha inhibition prevents neuronal NMDA receptor-stimulated arachidonic acid mobilization and prostaglandin production but not subsequent cell death. J Neurochem. 2008; 106(4):1828–1840. PMID: 18564366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effects of MK-801 (Dizocilpine) on Bilirubin Neurotoxicity in Mouse Cortical Cell Culture
- Effect of Etomidate on N-methyl-D-aspartate Induced Neurotoxicity in Rat Cortical Neurons
- Comparison of neurotoxicity induced by some glutathione depletors in mouse cortical cell cultures
- Cytoprotective effects of dihydrolipoic acid and lipoic acid on the oxidative stress in cultured rat cortical neurons
- Nitric Oxide-Mediated Cytotoxicity of Manganese in Basal Ganglia Neuronal Cells