J Korean Med Sci.
2004 Jun;19(3):374-383. 10.3346/jkms.2004.19.3.374.
Effect of Antisense TGF-beta1 Oligodeoxynucleotides in Streptozotocin-Induced Diabetic Rat Kidney
- Affiliations
-
- 1Department of Anatomy, Keimyung University School of Medicine, Daegu, Korea.
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea. park1234@dsmc.or.kr
- 3Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 4Kidney Institute, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 1786815
- DOI: http://doi.org/10.3346/jkms.2004.19.3.374
Abstract
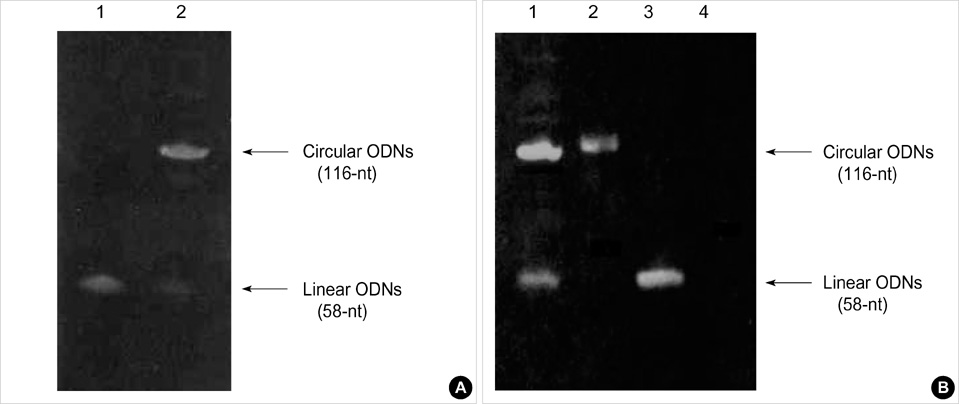
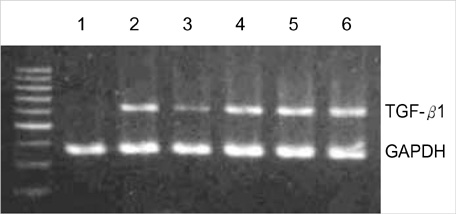
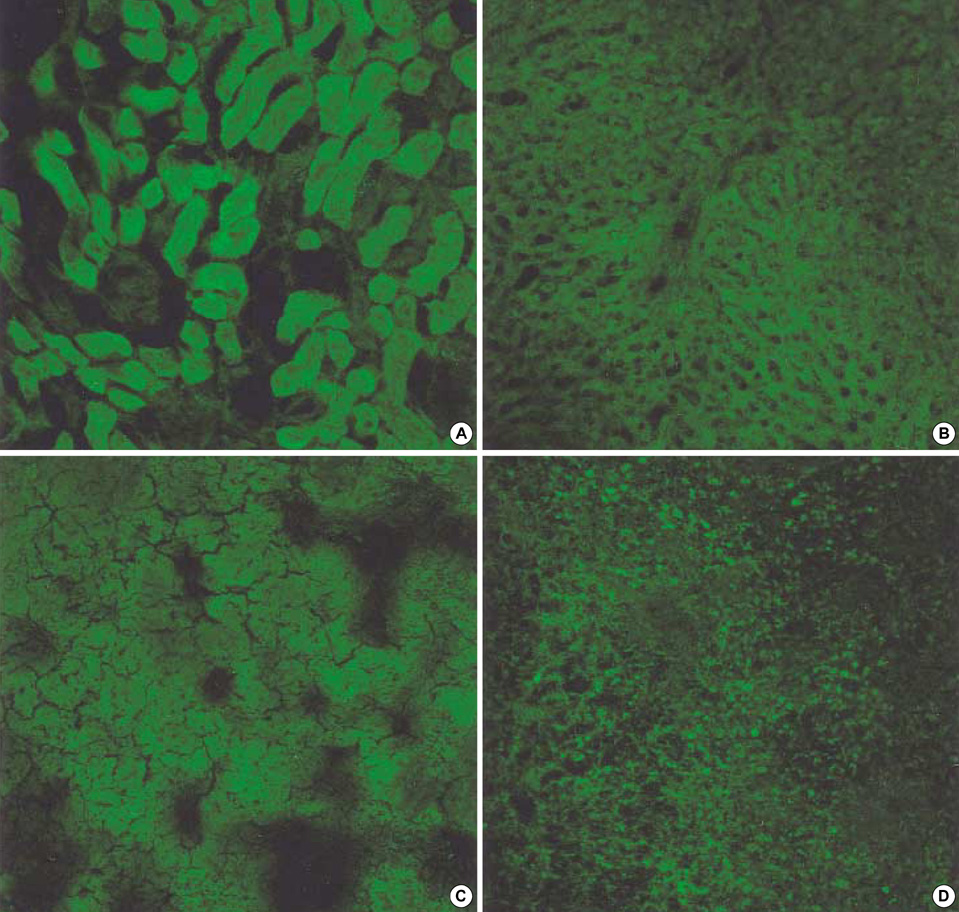
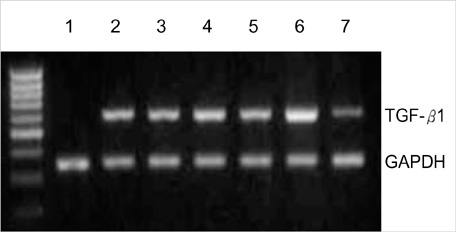
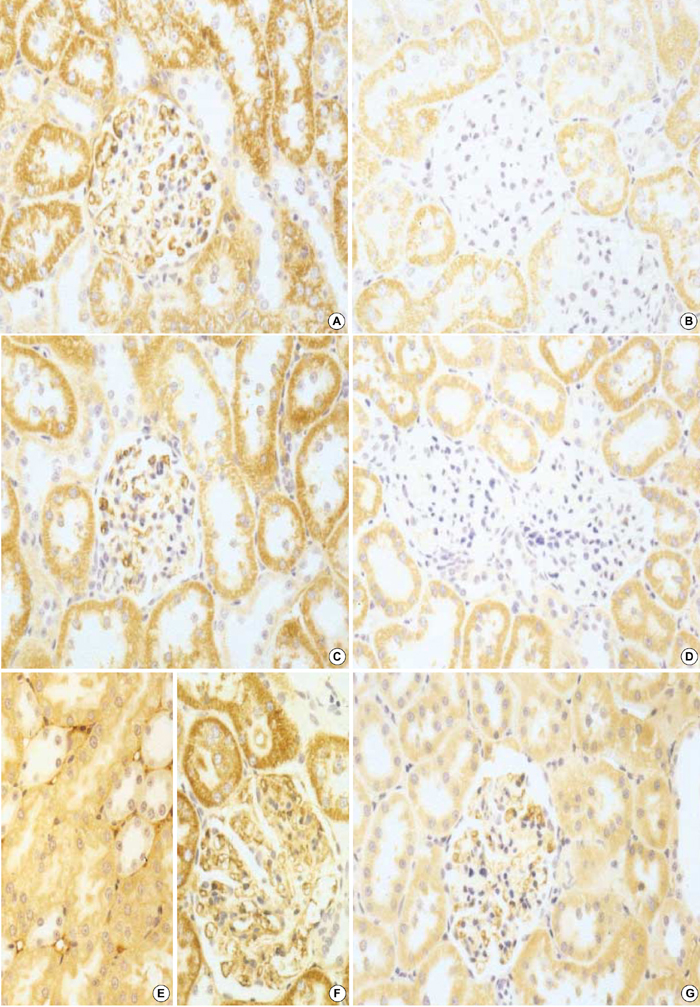
- Transforming growth factor (TGF)-beta1 is an important fibrogenic factor that is involved in the pathogenesis of diabetic nephropathy. We evaluated the effect of circular antisense TGF-beta1 oligodeoxynucleotides (ODNs) on the TGF-beta1 expression in the rat mesangial cell culture and in streptozotocin (STZ)-induced diabetic rats. Circular antisense TGF-beta1 ODNs were found to be stable in rat serum, significantly decreasing TGF-beta1 mRNA expression compared with linear antisense ODNs in the rat mesangial cell culture. Circular antisense TGF-beta1 ODNs were introduced into the tail vein of normal rats using hemagglutinating virus of Japan (HVJ)-liposome-mediated gene transfer method and were confirmed to be delivered effectively into the kidney, liver, lungs, and spleen. To inhibit the overexpression of TGF-beta1 in diabetic kidneys, we introduced circular antisense TGF-beta1 ODNs into the STZ-induced diabetic rats. On day 13 after circular antisense TGF-beta1 ODNs injection, TGF-beta1 mRNA and protein expression markedly decreased and urinary TGF-beta1 excretion rate also dropped in the circular antisense TGF-beta1 ODNs-treated diabetic rats. These results suggest that circular antisense TGF-beta1 ODNs may be a useful tool for developing new therapeutic application for progressive diabetic nephropathy.
MeSH Terms
-
Animals
Blood Glucose/metabolism
Cells, Cultured
Diabetes Mellitus, Experimental/*therapy
Enzyme-Linked Immunosorbent Assay
Gene Transfer Techniques
Immunohistochemistry
Liposomes/chemistry
Male
Microscopy, Confocal
Oligonucleotides, Antisense/metabolism/pharmacology/*therapeutic use
RNA/metabolism
RNA, Messenger/metabolism
Rats
Rats, Sprague-Dawley
Reverse Transcriptase Polymerase Chain Reaction
Streptozocin
Support, Non-U.S. Gov't
Time Factors
Transfection
Transforming Growth Factor beta/*genetics
Figure
Reference
-
1. Ziyadeh FN, Goldfarb S. The renal tubulointerstitium in diabetes mellitus. Kidney Int. 1991. 39:464–475.
Article2. Ziyadeh FN. Renal tubular basement membrane and collagen type IV in diabetes mellitus. Kidney Int. 1993. 43:114–120.
Article3. Ziyadeh FN. The Extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993. 22:736–744.
Article4. Bertoluci MC, Schmid H, Lachat JJ, Coimbra TM. Transforming growth factor-beta in the development of rat diabetic nephropathy. A 10-month study with insulin-treated rats. Nephron. 1997. 74:189–196.5. Rocco MV, Chen Y, Goldfarb S, Ziyadeh FN. Elevated glucose stimulates TGF-β gene expression and bioactivity in proximal tubule. Kidney Int. 1992. 41:107–114.
Article6. Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney Int. 1992. 42:647–656.
Article7. Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest. 1994. 93:536–542.8. Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN. Transcriptional activation of transforming growth factor-β1 in mesangial cell culture by high glucose concentration. Kidney Int. 1998. 54:1107–1116.
Article9. Oh JH, Ha HJ, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-β1 and fibronectin synthesis. Kidney Int. 1998. 54:1872–1878.
Article10. Ihm CG, Lee GS, Nast CC, Artishevsky A, Guillermo R, Levin PS, Glassock RJ, Adler SG. Early increased renal procollagen α1 (IV) mRNA levels in streptozotocin induced diabetes. Kidney Int. 1992. 41:768–777.11. Bollineni JS, Reddi AS. Transforming growth factor-β1 enhances glomerular collagen synthesis in diabetic rats. Diabetes. 1993. 42:1673–1677.
Article12. Shankland SJ, Scholey JW, Ly H, Thai K. Expression of transforming growth factor-β1 during diabetic renal hypertrophy. Kidney Int. 1994. 46:430–442.
Article13. Park IS, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor β and type IV collagen in early streptozotoc-ininduced diabetes. Diabetes. 1997. 46:473–480.14. Hill C, Flyvbjerg A, Gronbaek H, Petrik J, Hill DJ, Thomas CR, Sheppard MC, Logan A. The renal expression of transforming growth factor-β isoforms and their receptors in acute and chronic experimental diabetes in rats. Endocrinology. 2000. 141:1196–1208.
Article15. Roberts AB, McCune BK, Sporn MB. TGF-β: regulation of extracellular matrix. Kidney Int. 1992. 41:557–559.
Article16. Border WA, Noble NA. Transforming growth factorβ in tissue fibrosis. N Engl J Med. 1994. 331:1286–1292.17. Branton MH, Kopp JB. TGF-β and fibrosis. Microbes and Infection. 1999. 1:1349–1365.
Article18. Border WA, Noble NA, Ketteler M. TGF-β: a cytokine mediator of glomerulosclerosis and a target for therapeutic intervention. Kidney Int Suppl. 1995. 49:S59–S61.19. Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996. 45:522–530.
Article20. Border WA, Noble NA. TGF-β in kidney fibrosis: a target for gene therapy. Kidney Int. 1997. 51:1388–1396.
Article21. Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN. Therapy with antisense TGF-β1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol Renal Physiol. 2000. 278:F628–F634.22. Gewirtz AM, Sokol DL, Ratajczak MZ. Nucleic acid therapeutics: state of the art and future prospects. Blood. 1998. 92:712–736.
Article23. Furdon PJ, Dominski Z, Kole R. RNase H cleavage of RNA hybridized to oligonucleotides containing methylphosphonate, phosphorothioate and phosphodiester bonds. Nucleic Acids Res. 1989. 17:9193–9204.
Article24. Beltinger C, Saragovi HU, Smith RM, LeSauteur L, Shah N, DeDionisio L, Christensen L, Raible A, Jarett L, Gewirtz AM. Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J Clin Invest. 1995. 95:1814–1823.
Article25. Agrawal S. Antisense oligonucleotides: towards clinical trials. Trends Biotechnol. 1996. 14:376–387.
Article26. Guvakova MA, Yakubov LA, Vlodavsky I, Tonkinson JL, Stein CA. Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J Biol Chem. 1995. 270:2620–2627.
Article27. Moon IJ, Choi KS, Choi YK, Kim JE, Lee YG, Schreiber AD, Park JG. Potent growth inhibition of leukemic cells by novel ribbon-type antisense oligonucleotides to c-myb1. J Biol Chem. 2000. 275:4647–4653.
Article28. Han SM, Kim EJ, Jeoung HS, Lee BY, Lee SS, Park KK, Kim HC. The effect of ribbon-type antisense oligodeoxynucleotides for transforming growth factor-β1 in unilateral ureteral obstruction. Korean J Pathol. 2002. 36:84–92.29. Leehey DJ, Song RH, Alavi N, Singh AK. Decreased degradative enzymes in mesangial cells cultured in high glucose media. Diabetes. 1995. 44:929–935.
Article30. Kaname S, Uchida S, Ogata E, Kurokawa K. Autocrine secretion of transforming growth factor-β in cultured rat mesangial cells. Kidney Int. 1992. 42:1319–1327.
Article31. Akagi Y, Isaka Y, Arai M, Kaneko T, Takenaka M, Moriyama T, Kaneda Y, Ando A, Orita Y, Kamada T, Ueda N, Imai E. Inhibition of TGF-β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996. 50:148–155.
Article32. Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-β in rats. Am J Physiol. 1998. 274:F635–F641.33. Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor-β1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000. 58:1885–1892.
Article34. Morishita R, Gibbons GH, Kaneda Y, Ogihara T, Dzau VJ. Systemic administration of HVJ viral coat-liposome complex containing human insulin vector decreases glucose level in diabetic mouse: a model of gene therapy. Biochem Biophys Res Commun. 2000. 273:666–674.
Article35. Agrawal S, Temsamani J, Tang JY. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci USA. 1991. 88:7595–7599.
Article36. Zhang R, Diasio RB, Lu Z, Liu T, Jiang Z, Galbraith WM, Agrawal S. Pharmacokinetics and tissue distribution in rats of an oligodeoxynucleotide phosphorothioate (GEM 91) developed as a therapeutic agent for human immunodeficiency virus type-1. Biochem Pharmacol. 1995. 49:929–939.
Article37. Kaneda Y, Saeki Y, Morishita R. Gene therapy using HVJ-liposomes: the best of both worlds? Mol Med Today. 1999. 5:298–303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Ribbon-Type Antisense Oligodeoxynucleotides for Transforming Growth Factor-beta1 in Unilateral Ureteral Obstruction
- Downregulation of TGF-beta1 Expression in Penile Corpus Cavernosum by Antisense TGF-beta1 Oligonucleotides in Diabetic Rat
- Attenuation of the Expression of Fibrogenic Molecules by Transforming Growth Factor-beta1 Antisense in Cultured Rat Mesangial Cells
- Effects of TGF-beta1 Ribbon Antisense on CCl4-induced Liver Fibrosis
- Plasminogen Activator Inhibitor-1 Antisense Oligodeoxynucleotides Abrogate Mesangial Fibronectin Accumulation