J Korean Med Sci.
2013 Dec;28(12):1723-1728. 10.3346/jkms.2013.28.12.1723.
Role of Regulatory T Cells in Transferable Immunological Tolerance to Bone Marrow Donor in Murine Mixed Chimerism Model
- Affiliations
-
- 1Department of Microbiology and Immunology, Translational Xenotransplantation Research Center, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. chgpark@snu.ac.kr
- KMID: 1779411
- DOI: http://doi.org/10.3346/jkms.2013.28.12.1723
Abstract
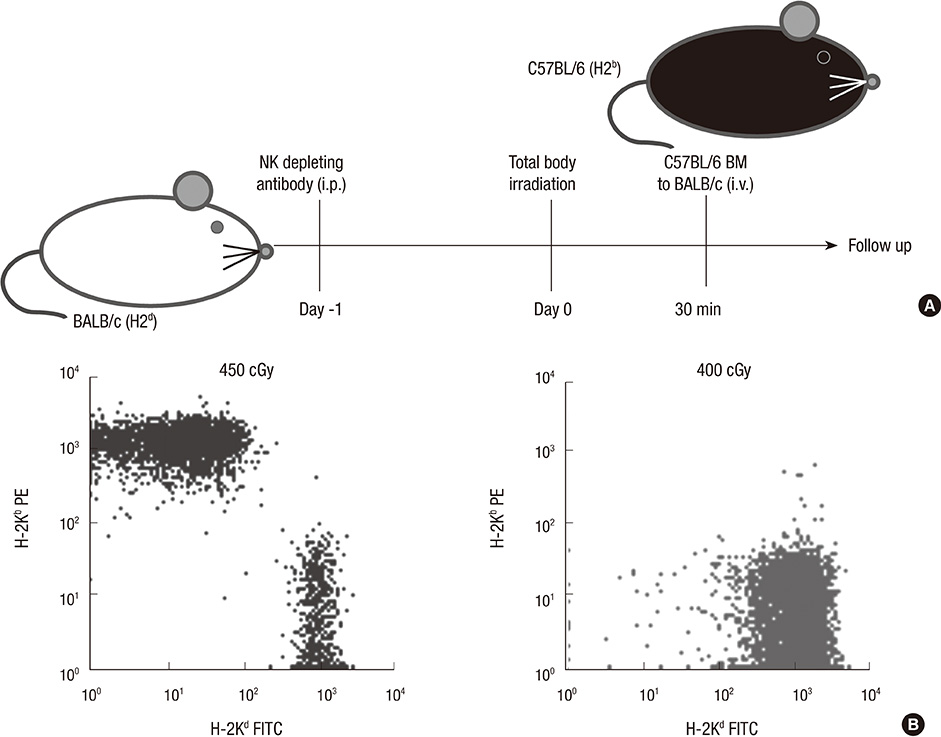
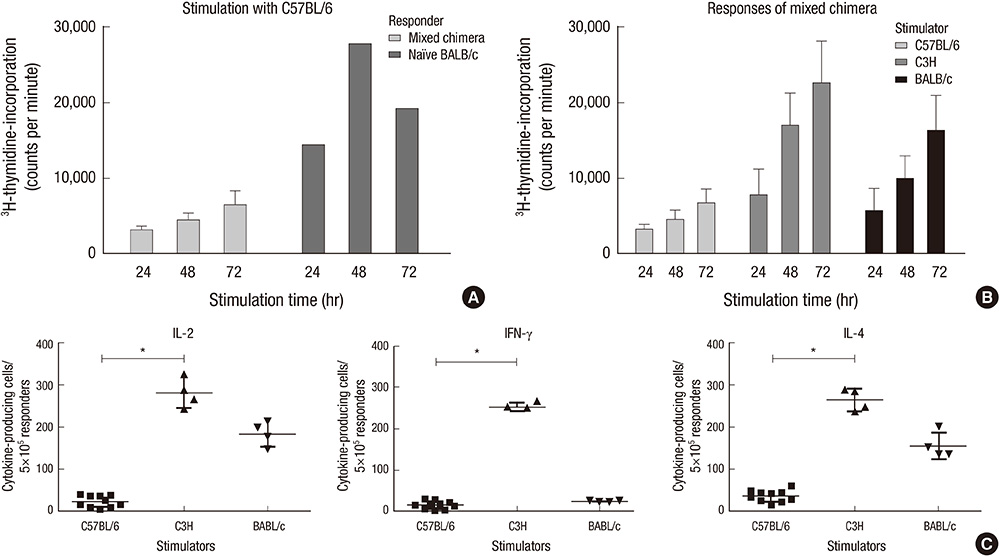
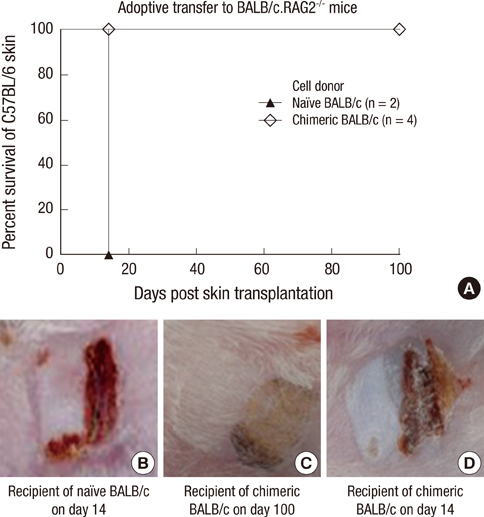
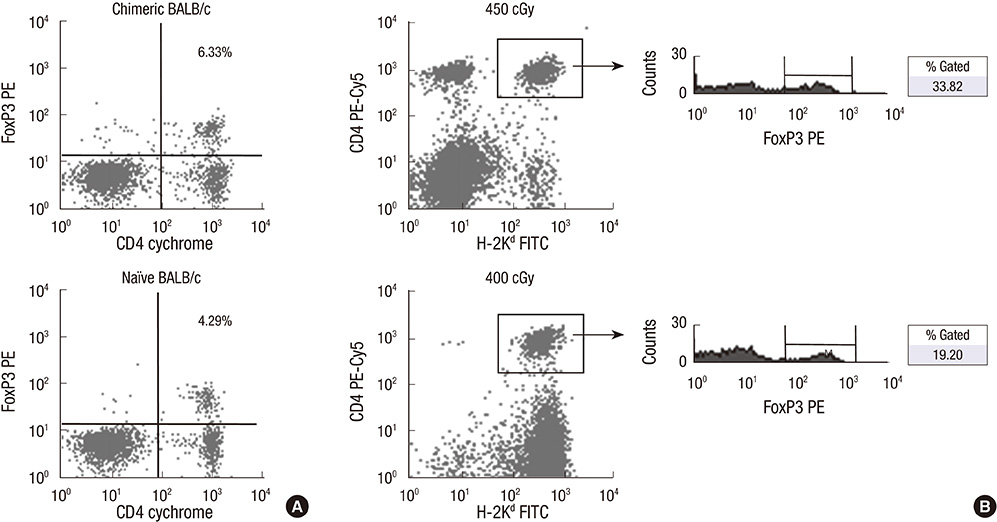
- Constructing a bone marrow chimera prior to graft transplantation can induce donor-specific immune tolerance. Mixed chimerism containing hematopoietic cells of both recipient- and donor-origin has advantages attributed from low dose of total body irradiation. In this study, we explored the mechanism of mixed chimerism supplemented with depletion of Natural Killer cells. Mixed chimerism with C57BL/6 bone marrow cells was induced in recipient BALB/c mice which were given 450 cGy of gamma-ray irradiation (n = 16). As revealed by reduced proliferation and cytokine production in mixed leukocyte reaction and ELISpot assay (24.6 vs 265.5), the allo-immune response to bone marrow donor was reduced. Furthermore, the induction of transferable immunological tolerance was confirmed by adoptive transfer and subsequent acceptance of C57BL/6 skin graft (n = 4). CD4+FoxP3+ regulatory T cells were increased in the recipient compartment of the mixed chimera (19.2% --> 33.8%). This suggests that regulatory T cells may be therapeutically used for the induction of graft-specific tolerance by mixed chimerism.
MeSH Terms
-
Animals
Bone Marrow Cells/cytology
*Bone Marrow Transplantation
Cell Proliferation
Chimerism
Cytokines/metabolism
Gamma Rays
Graft Survival
*Immune Tolerance
Killer Cells, Natural/immunology/radiation effects
Leukocytes/immunology/radiation effects
Mice
Mice, Inbred BALB C
Mice, Inbred C57BL
Models, Animal
Skin Transplantation
T-Lymphocytes, Regulatory/cytology/*immunology/metabolism
Whole-Body Irradiation
Cytokines
Figure
Reference
-
1. Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012; 12:417–430.2. Zelenika D, Adams E, Humm S, Lin CY, Waldmann H, Cobbold SP. The role of CD4+ T-cell subsets in determining transplantation rejection or tolerance. Immunol Rev. 2001; 182:164–179.3. Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008; 3:189–220.4. Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999; 68:459–467.5. Sachs DH. Tolerance: of mice and men. J Clin Invest. 2003; 111:1819–1821.6. Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat Rev Nephrol. 2010; 6:594–605.7. Pilat N, Hock K, Wekerle T. Mixed chimerism through donor bone marrow transplantation: a tolerogenic cell therapy for application in organ transplantation. Curr Opin Organ Transplant. 2012; 17:63–70.8. Park M, Koh KN, Seo JJ, Im HJ. Clinical implications of chimerism after allogeneic hematopoietic stem cell transplantation in children with non-malignant diseases. Korean J Hematol. 2011; 46:258–264.9. Salama AD, Remuzzi G, Harmon WE, Sayegh MH. Challenges to achieving clinical transplantation tolerance. J Clin Invest. 2001; 108:943–948.10. Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Sachs DH. Characterization of mixed allogeneic chimeras: immunocompetence, in vitro reactivity, and genetic specificity of tolerance. J Exp Med. 1985; 162:231–244.11. Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells: differences in kinetics and target antigens recognized. J Exp Med. 1987; 166:1499–1509.12. Cho SG, Shuto Y, Soda Y, Nakazaki Y, Izawa K, Uchimaru K, Takahashi S, Tani K, Tojo A, Asano S. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol. 2004; 32:1246–1254.13. Kasai M, Yoneda T, Habu S, Maruyama Y, Okumura K, Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981; 291:334–335.14. Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007; 27:203–213.15. Kean LS, Hamby K, Koehn B, Lee E, Coley S, Stempora L, Adams AB, Heiss E, Pearson TC, Larsen CP. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant. 2006; 6:292–304.16. Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. "Infectious" transplantation tolerance. Science. 1993; 259:974–977.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Immune Tolerance Effect of Donor Chimerism Induced by Sublethal dose of Radiation on Allogeneic Organ Transplantation
- Effects on tolerance of chimerism and graft-versus-host disease in vascularized bone marrow allotransplantation
- Practical Evaluation of Engraftment and Mixed Chimerism Using PCR Amplification of a Microsatellite in the Class II Eb Gene in Murine MHC-mismatched, Nonmyeloablative Bone Marrow Transplantation
- Prevention of Allograft Rejection by Immune Tolerance
- Clinical Strategies to Develop Transplantation Tolerance