Korean J Hematol.
2007 Jun;42(2):91-97. 10.5045/kjh.2007.42.2.91.
Practical Evaluation of Engraftment and Mixed Chimerism Using PCR Amplification of a Microsatellite in the Class II Eb Gene in Murine MHC-mismatched, Nonmyeloablative Bone Marrow Transplantation
- Affiliations
-
- 1Division of Oncology, Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Rhematism Research Center, Catholic Institute of Medical Sciences, The Catholic University of Korea, Seoul, Korea.
- 3Catholic Hematopoietic Stem Cell Transplantation Center, The Catholic University of Korea, Seoul, Korea. chosg@catholic.ac.kr
- 4Department of Radiation Oncology, The Catholic University of Korea, Seoul, Korea.
- KMID: 2083505
- DOI: http://doi.org/10.5045/kjh.2007.42.2.91
Abstract
-
BACKGROUND: Although engraftment following murine allogeneic bone marrow transplantation (BMT) is most commonly confirmed by H2 typing using flow cytometry, recipient mice can be seriously injured during peripheral blood (PB) sampling. Therefore, we developed an alternative DNA-based assay that does not require the large volume of PB necessary for flow cytometry.
METHODS
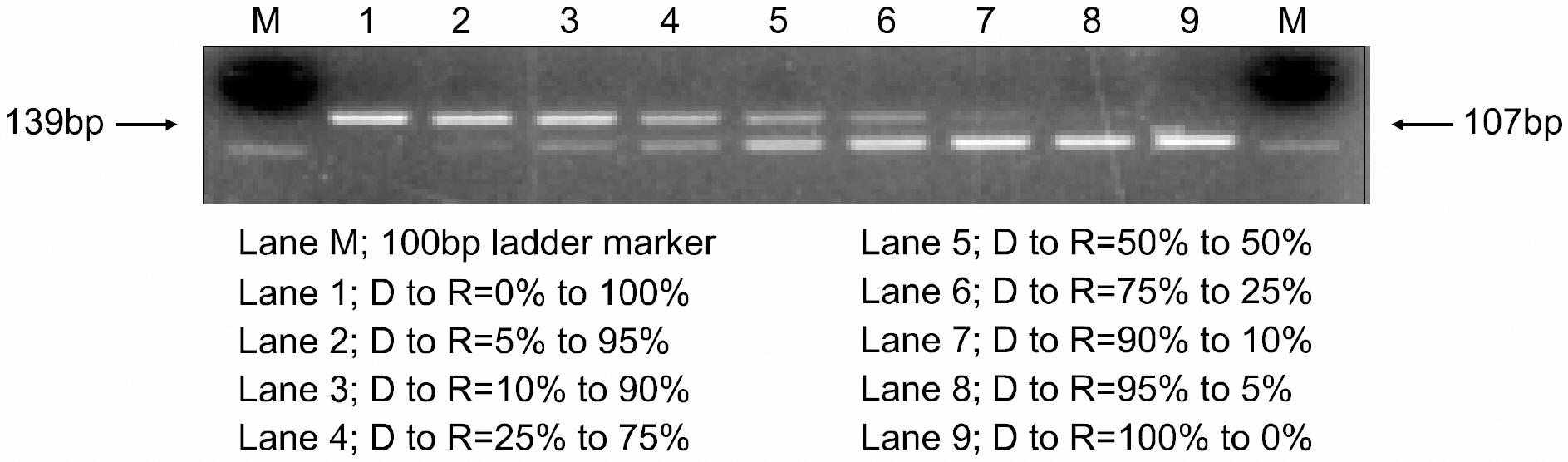
A minute volume of PB from the tail vein was used to evaluate the engraftment by PCR amplification of a microsatellite in the class II Eb gene. Dilution experiments were performed to evaluate the sensitivity of this assay for detecting donor cells in mixed cell populations compared with flow cytometry analysis.
RESULTS
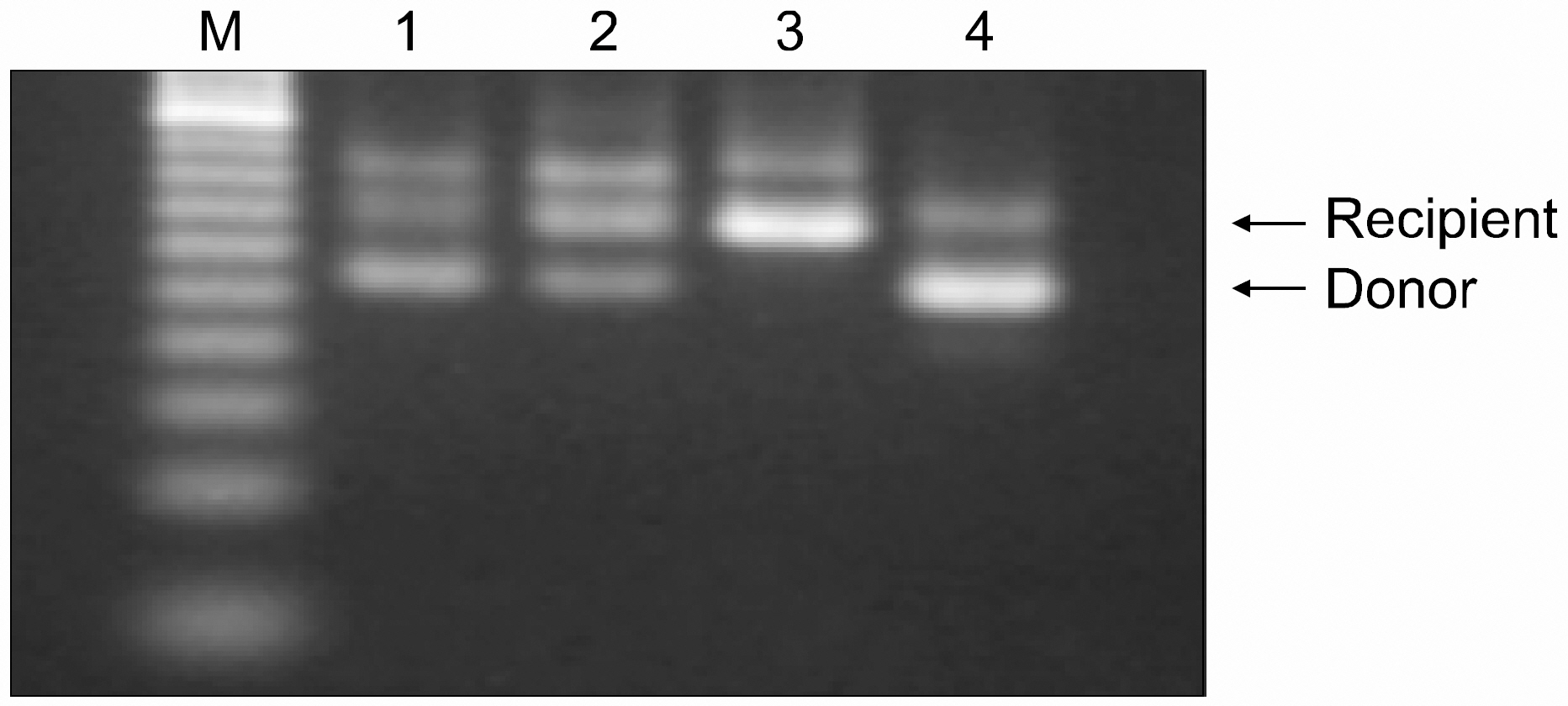
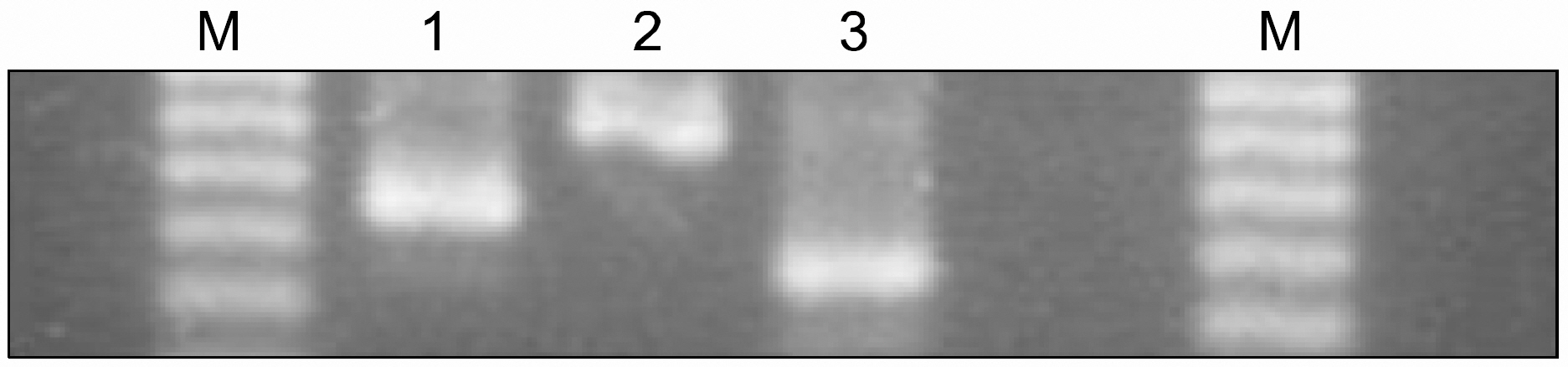
Early engraftment and mixed chimerism were confirmed, based on the length variation of the microsatellite in the class II Eb gene. The degree of donor chimerism in the donor-recipient cell mixture could be estimated semiquantitatively in a dilution experiment. The sensitivity of this assay by the naked eye approached 10% of the degree of donor chimerism.
CONCLUSION
PCR amplification of a microsatellite in the class II Eb gene can be a useful alternative to flow cytometry for evaluating early engraftment and mixed chimerism following murine nonmyeloablative BMT.
Keyword
MeSH Terms
Figure
Reference
-
1). Exner BG., Domenick MA., Bergheim M., Mueller YM., Ildstad ST. Clinical applications of mixed chimerism. Ann N Y Acad Sci. 1999. 872:377–85. discussion 385-6.
Article2). Cho SG., Shuto Y., Soda Y, et al. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol. 2004. 32:1246–54.
Article3). Mapara MY., Kim YM., Wang SP., Bronson R., Sachs DH., Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002. 100:1903–9.
Article4). Luznik L., Slansky JE., Jalla S, et al. Successful therapy of metastatic cancer using tumor vaccines in mixed allogeneic bone marrow chimeras. Blood. 2003. 101:1645–52.
Article5). Exner BG., Acholonu IN., Bergheim M., Mueller YM., Ildstand ST. Mixed allogeneic chimerism to induce tolerance to solid organ and cellular grafts. Acta Haematol. 1999. 101:78–81.
Article6). Mapara MY., Kim YM., Marx J., Sykes M. Donor lymphocyte infusion-mediated graft-versus-leukemia effects in mixed chimeras established with a non-myeloablative conditioning regimen: extinction of graft-versus-leukemia effects after conversion to full donor chimerism. Transplantation. 2003. 76:297–305.7). Sykes M., Preffer F., McAfee S, et al. Mixed lympho-haemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet. 1999. 353:1755–9.8). Li H., Kaufman CL., Boggs SS., Johnson PC., Patrene KD., Ildstad ST. Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in nonobese diabetic (NOD) mice. J Immunol. 1996. 156:380–8.9). Nikolic B., Takeuchi Y., Leykin I., Fudaba Y., Smith RN., Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes. 2004. 53:376–83.
Article10). Cho SG., Min SY., Park MJ, et al. Immunoregulatory effects of allogeneic mixed chimerism induced by nonmyeloablative bone marrow transplantation on chronic inflammatory arthritis and autoimmunity in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2006. 54:1878–87.
Article11). Saha BK. Typing of murine major histocompatibility complex with a microsatellite in the class II Eb gene. J Immunol Methods. 1996. 194:77–83. Acad Sci U S A 1993;90: 5312-6.
Article12). Saha BK., Shields JJ., Miller RD., Hansen TH., Shreffler DC. A highly polymorphic microsatellite in the class II Eb gene allows tracing of major histocompatibility complex evolution in mouse. Proc Natl.13). Gardiner N., Lawler M., O'Riordan J., DeArce M., Humphries P., McCann SR. Persistent donor chimaerism is consistent with disease-free survival following BMT for chronic myeloid leukaemia. Bone Marrow Transplant. 1997. 20:235–41.
Article14). Gardiner N., Lawler M., O'Riordan J., De'Arce M., McCann SR. Donor chimaerism is a strong indicator of disease free survival following bone marrow transplantation for chronic myeloid leukaemia. Leukemia. 1997. 11(Suppl 3):512–5.15). Lawler M., Humphries P., McCann SR. Evaluation of mixed chimerism by in vitro amplification of dinucleotide repeat sequences using the polymerase chain reaction. Blood. 1991. 77:2504–14.
Article16). Park SJ., Min WS., Yang IH, et al. Effects of mixed chimerism and immune modulation on GVHD, disease recurrence and survival after HLA-identical marrow transplantation for hematologic malignancies. Korean J Intern Med. 2000. 15:224–31.17). Buno I., Nava P., Simon A, et al. A comparison of fluorescent in situ hybridization and multiplex short tandem repeat polymerase chain reaction for quantifying chimerism after stem cell transplantation. Haematologica. 2005. 90:1373–9.18). O'Neill PA., Lawler M., Pullens R, et al. PCR amplification of short tandem repeat sequences allows serial studies of chimaerism/engraftment following BMT in rodents. Bone Marrow Transplant. 1996. 17:265–71.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Mixed Chimerism after Sex-Mismatched Bone Marrow Transplantation Using Fluorescence in situ Hybridization: Engraftment and Follow-up Evaluation
- The effects of mixed chimerism conducted by natural killer cell depletion with non myeloablation on islet allograft rejection
- Hematopoietic Stem Cell Transplantation Using Non-myeloablative Preparative Regimens
- Fluorescence in situ Hybridization using Chromosome X alpha-Satellite Probe To Evaluate Engraftment and To Monitor Residual Disease after Bone Marrow Transplantation
- Induction of Tolerance to Complete Histocompatibility Mismatched Mice Islets through the Co-transplantation of Bone Marrow Cells in a Minimal Nonmyeloablative Condition