J Korean Med Sci.
2011 Apr;26(4):482-491. 10.3346/jkms.2011.26.4.482.
Establishment of Efficacy and Safety Assessment of Human Adipose Tissue-Derived Mesenchymal Stem Cells (hATMSCs) in a Nude Rat Femoral Segmental Defect Model
- Affiliations
-
- 1Graduate School of Immunology, Seoul National University College of Medicine, Seoul, Korea. bckang@snu.ac.kr
- 2Department of Experimental Animal Research, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Stem Cell Research Center, RNL Bio Co., Ltd., Seoul, Korea.
- KMID: 1777877
- DOI: http://doi.org/10.3346/jkms.2011.26.4.482
Abstract
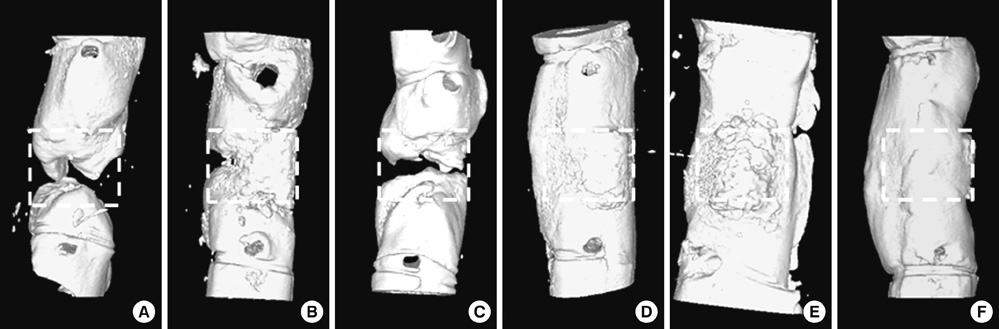
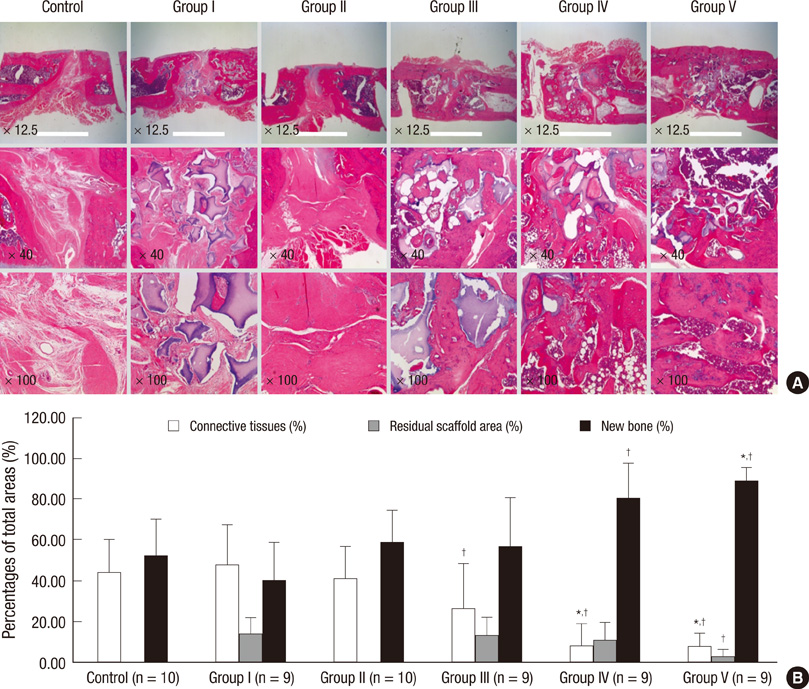
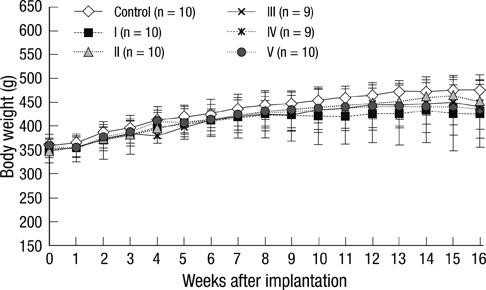
- Human adipose tissue-derived mesenchymal stem cell (hATMSC) have emerged as a potentially powerful tool for bone repair, but an appropriate evaluation system has not been established. The purpose of this study was to establish a preclinical assessment system to evaluate the efficacy and safety of cell therapies in a nude rat bone defect model. Segmental defects (5 mm) were created in the femoral diaphyses and transplanted with cell media (control), hydroxyapatite/tricalcium phosphate scaffolds (HA/TCP, Group I), hATMSCs (Group II), or three cell-loading density of hATMSC-loaded HA/TCP (Group III-V). Healing response was evaluated by serial radiography, micro-computed tomography and histology at 16 weeks. To address safety-concerns, we conducted a GLP-compliant toxicity study. Scanning electron microscopy studies showed that hATMSCs filled the pores/surfaces of scaffolds in a cell-loading density-dependent manner. We detected significant increases in bone formation in the hATMSC-loaded HA/TCP groups compared with other groups. The amount of new bone formation increased with increases in loaded cell number. In a toxicity study, no significant hATMSC-related changes were found in body weights, clinical signs, hematological/biochemical values, organ weights, or histopathological findings. In conclusion, hATMSCs loaded on HA/TCP enhance the repair of bone defects and was found to be safe under our preclinical efficacy/safety hybrid assessment system.
Keyword
MeSH Terms
-
Adipose Tissue/*cytology
Animals
Biocompatible Materials/therapeutic use
Bone Diseases/pathology/radiography/*therapy
Bone Regeneration/physiology
Calcium Phosphates/therapeutic use
Diaphyses/radiography/surgery/ultrastructure
Disease Models, Animal
Durapatite/therapeutic use
Femur/*pathology/radiography/surgery
Humans
Male
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stem Cells/*cytology
Rats
Rats, Nude
Tissue Engineering
Tomography, X-Ray Computed
Transplantation, Heterologous
Figure
Reference
-
1. Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Miyamoto S, Nozaki K, Takaoka K. Biodegradable poly-D,L-lactic acid-polyethylene glycol block copolymers as a BMP delivery system for inducing bone. J Bone Joint Surg Am. 2001. 83-A:Suppl 1. S92–S98.2. Ikeuchi M, Dohi Y, Horiuchi K, Ohgushi H, Noshi T, Yoshikawa T, Yamamoto K, Sugimura M. Recombinant human bone morphogenetic protein-2 promotes osteogenesis within atelopeptide type I collagen solution by combination with rat cultured marrow cells. J Biomed Mater Res. 2002. 60:61–69.3. Boo JS, Yamada Y, Okazaki Y, Hibino Y, Okada K, Hata K, Yoshikawa T, Sugiura Y, Ueda M. Tissue-engineered bone using mesenchymal stem cells and a biodegradable scaffold. J Craniofac Surg. 2002. 13:231–239.4. Dong J, Uemura T, Shirasaki Y, Tateishi T. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002. 23:4493–4502.5. Tancred DC, Carr AJ, McCormack BA. Development of a new synthetic bone graft. J Mater Sci Mater Med. 1998. 9:819–823.6. Walsh WR, Vizesi F, Michael D, Auld J, Langdown A, Oliver R, Yu Y, Irie H, Bruce W. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008. 29:266–271.7. Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007. 25:371–379.8. Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, Huso DL, Semenza GL. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006. 66:10849–10854.9. Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007. 25:1586–1594.10. Tasso R, Augello A, Carida M, Postiglione F, Tibiletti MG, Bernasconi B, Astigiano S, Fais F, Truini M, Cancedda R, Pennesi G. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009. 30:150–157.11. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998. 16:155–162.12. Muraglia A, Corsi A, Riminucci M, Mastrogiacomo M, Cancedda R, Bianco P, Quarto R. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003. 116:2949–2955.13. Kadiyala S, Jaiswal N, Bruder S. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997. 3:173–185.14. Wan C, He Q, Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res. 2006. 24:610–618.15. Xia Z, Ye H, Choong C, Ferguson DJ, Platt N, Cui Z, Triffitt JT. Macrophagic response to human mesenchymal stem cell and poly (epsilon-caprolactone) implantation in nonobese diabetic/severe combined immunodeficient mice. J Biomed Mater Res A. 2004. 71:538–548.16. Jager M, Degistirici O, Knipper A, Fischer J, Sager M, Krauspe R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J Bone Miner Res. 2007. 22:1224–1233.17. Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, Esser S. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004. 6:212–222.18. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.19. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003. 31:890–896.20. Kim WS, Kim HK. Tissue engineered vascularized bone formation using in vivo implanted osteoblast-polyglycolic acid scaffold. J Korean Med Sci. 2005. 20:479–482.21. Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989. 23:883–894.22. Nery EB, Lynch KL, Hirthe WM, Mueller KH. Bioceramic implants in surgically produced infrabony defects. J Periodontol. 1975. 46:328–347.23. Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O'Byrne E, Pellas TC. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002. 23:109–119.24. Tatebe M, Nakamura R, Kagami H, Okada K, Ueda M. Differentiation of transplanted mesenchymal stem cells in a large osteochondral defect in rabbit. Cytotherapy. 2005. 7:520–530.25. Shao X, Goh JC, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006. 12:1539–1551.26. Yamazoe K, Mishima H, Torigoe K, Iijima H, Watanabe K, Sakai H, Kudo T. Effects of atelocollagen gel containing bone marrow-derived stromal cells on repair of osteochondral defect in a dog. J Vet Med Sci. 2007. 69:835–839.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue
- Adipose-derived stem cells: characterization and clinical application
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro