J Korean Med Assoc.
2012 Aug;55(8):757-769. 10.5124/jkma.2012.55.8.757.
Adipose-derived stem cells: characterization and clinical application
- Affiliations
-
- 1Department of Plastic Surgery, Seoul St. Mary Hospital, The Catholic University of Korea, Seoul, Korea. rhie@catholic.ac.kr
- KMID: 1435182
- DOI: http://doi.org/10.5124/jkma.2012.55.8.757
Abstract
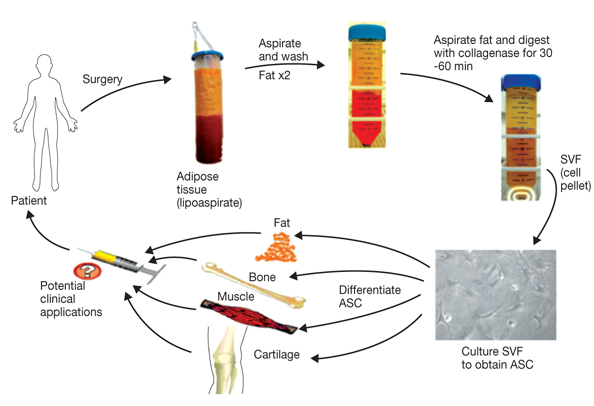
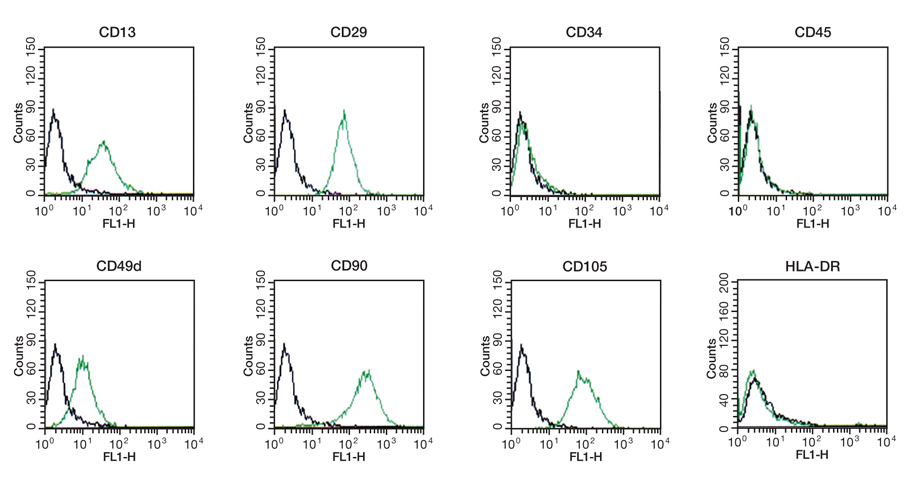
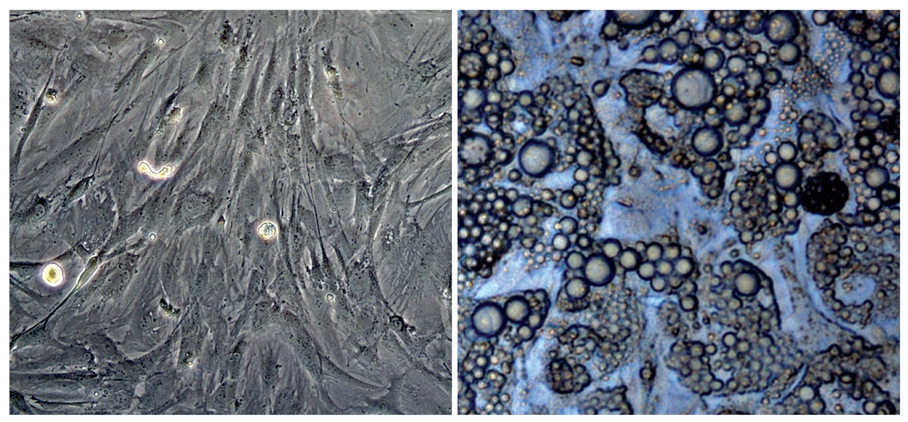
- Adipose tissue is an ideal tissue to use as an autologous substitute with which to approach tissue deficiency. Clinically, the use of fat grafts and adipose-derived stem cells has dramatically increased worldwide for reconstructive and aesthetic purposes. Human adipose tissue contains a population of pluripotent stem cells capable of differentiating along multiple mesenchymal cell lineages. Adipose tissue is an abundant, expendable, and easily obtained tissue that may prove to be an ideal source of autologous stem cells for regenerating tissues. The recent identification and characterization of multilineage cells from human adipose tissue has been met with a great deal of excitement by the field of tissue engineering. The authors' laboratory has characterized a population of cells obtained from human adipose tissue that have the capacity to differentiate into osteoblasts, chondrocytes, adipocytes, and neuron-like cells in vitro. This article summarizes the basic study of the adipose tissue as a multipotential stem cell source of tissue engineering techniques that are currently being developed to solve common aesthetic problems.
MeSH Terms
Figure
Cited by 1 articles
-
The principles of tissue engineering and its recent advances and future prospects
Woo Seob Kim
J Korean Med Assoc. 2014;57(2):145-154. doi: 10.5124/jkma.2014.57.2.145.
Reference
-
1. Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J. 1995. 309(Pt 3):697–710.
Article2. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article3. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.4. Young C, Jarrell BE, Hoying JB, Williams SK. A porcine model for adipose tissue-derived endothelial cell transplantation. Cell Transplant. 1992. 1:293–298.5. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001. 189:54–63.
Article6. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adi-pose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
Article7. Zuk PA. Consensus statement. International Fat Applied Technology Society 2nd international meeting. 2004. Pittsburg: International Fat Applied Technology Society.
Article8. Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004. 6:7–14.
Article9. Boquest AC, Shahdadfar A, Brinchmann JE, Collas P. Isolation of stromal stem cells from human adipose tissue. Methods Mol Biol. 2006. 325:35–46.
Article10. Parker AM, Katz AJ. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin Biol Ther. 2006. 6:567–578.
Article11. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006. 24:376–385.
Article12. Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000. 26:1159–1166.
Article13. Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009. 79:235–244.
Article14. Safford KM, Rice HE. Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005. 6:57–62.
Article15. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.
Article16. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003. 5:362–369.
Article17. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005. 54:132–141.
Article18. Boone C, Mourot J, Gregoire F, Remacle C. The adipose conversion process: regulation by extracellular and intracellular factors. Reprod Nutr Dev. 2000. 40:325–358.
Article19. Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003. (412):196–212.
Article20. Lee JA, Parrett BM, Conejero JA, Laser J, Chen J, Kogon AJ, Nanda D, Grant RT, Breitbart AS. Biological alchemy: engineering bone and fat from fat-derived stem cells. Ann Plast Surg. 2003. 50:610–617.
Article21. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003. 58:137–160.
Article22. Tholpady SS, Llull R, Ogle RC, Rubin JP, Futrell JW, Katz AJ. Adipose tissue: stem cells and beyond. Clin Plast Surg. 2006. 33:55–62. vi
Article23. Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003. 34:565–571.
Article24. Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003. 3:705–713.
Article25. Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004. 110:349–355.
Article26. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.
Article27. American Society for Aesthetic Plastic Surgery. Cosmetic surgery quick facts: 2008 ASAPS statistics [Internet]. 2009. cited 2012 May 18. New York: American Society for Aesthetic Plastic Surgery;Available from: http://www.surgery.org.28. Stosich MS, Bastian B, Marion NW, Clark PA, Reilly G, Mao JJ. Vascularized adipose tissue grafts from human mesenchymal stem cells with bioactive cues and microchannel conduits. Tissue Eng. 2007. 13:2881–2890.29. Halberstadt C, Austin C, Rowley J, Culberson C, Loebsack A, Wyatt S, Coleman S, Blacksten L, Burg K, Mooney D, Holder W Jr. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Eng. 2002. 8:309–319.
Article30. Kim DH, Je CM, Sin JY, Jung JS. Effect of partial hepatectomy on in vivo engraftment after intravenous administration of human adipose tissue stromal cells in mouse. Microsurgery. 2003. 23:424–431.
Article31. Rophael JA, Craft RO, Palmer JA, Hussey AJ, Thomas GP, Morrison WA, Penington AJ, Mitchell GM. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am J Pathol. 2007. 171:2048–2057.
Article32. Nathan S, Das De S, Thambyah A, Fen C, Goh J, Lee EH. Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng. 2003. 9:733–744.
Article33. Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003. 48:418–429.
Article34. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004. 22:560–567.
Article35. Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004. 32:370–373.
Article36. Duplomb L, Dagouassat M, Jourdon P, Heymann D. Differentiation of osteoblasts from mouse embryonic stem cells without generation of embryoid body. In Vitro Cell Dev Biol Anim. 2007. 43:21–24.
Article37. Knippenberg M, Helder MN, Zandieh Doulabi B, Wuisman PI, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006. 342:902–908.
Article38. Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, Ailhaud G, Dani C. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005. 201:1397–1405.
Article39. Jun YJ, Rhie JW, Choi YS, Kim YJ, Kim SE, Lee JI, Han KT. The effects of adipose derived stem cells on neurogenic differentiation and induction of nerve regeneration. J Korean Soc Plast Reconstr Surg. 2006. 33:205–212.
Article40. Williams SK, Wang TF, Castrillo R, Jarrell BE. Liposuction-derived human fat used for vascular graft sodding contains endothelial cells and not mesothelial cells as the major cell type. J Vasc Surg. 1994. 19:916–923.
Article41. Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003. 301:1016–1022.
Article42. Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007. 119:1409–1422.
Article43. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005. 129:118–129.
Article44. Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005. 16:1131–1141.
Article45. Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adi-pose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005. 332:370–379.
Article46. Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005. 48:1416–1423.
Article47. Garcia-Olmo D, Garcia-Arranz M, Garcia LG, Cuellar ES, Blanco IF, Prianes LA, Montes JA, Pinto FL, Marcos DH, Garcia-Sancho L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell-based therapy. Int J Colorectal Dis. 2003. 18:451–454.
Article48. Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003. 183:355–366.
Article49. Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Muller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006. 341:1135–1140.
Article50. De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003. 89:267–270.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose Tissue Derived Mesenchymal Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose Tissue-Derived Stem Cells for Myocardial Regeneration
- Brief Review of Adipose Derived Cells
- Characterization of multipotent mesenchymal stem cells isolated from adipose tissue and bone marrow in pigs