Ann Dermatol.
2011 May;23(2):150-155. 10.5021/ad.2011.23.2.150.
Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- Affiliations
-
- 1Department of Dermatology, Dongguk University Ilsan Hospital, Goyang, Korea.
- 2Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea. khcho@snu.ac.kr
- KMID: 2156655
- DOI: http://doi.org/10.5021/ad.2011.23.2.150
Abstract
- BACKGROUND
Despite numerous treatments available for deteriorated cutaneous wound healing such as a diabetic foot, there is still the need for more effective therapy. Adipose-derived stem cells (ASCs) are mesenchymal stem cells, which are self-renewing and multipotent. Mesenchymal stem cells have the potential for tissue repair and regeneration.
OBJECTIVE
To investigate the effects of human ASCs on the healing of cutaneous wounds in nude mice.
METHODS
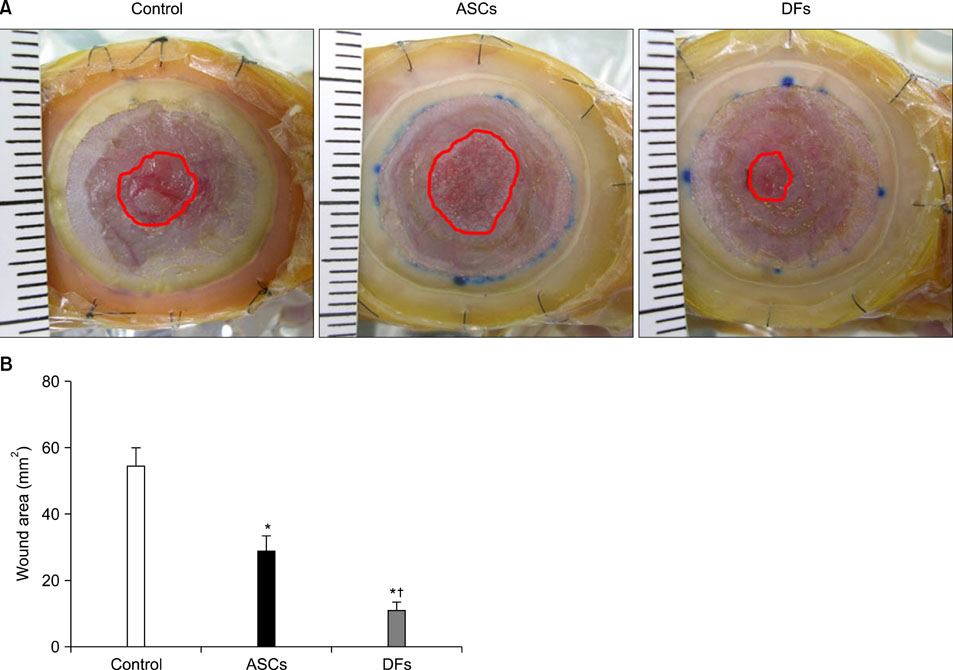
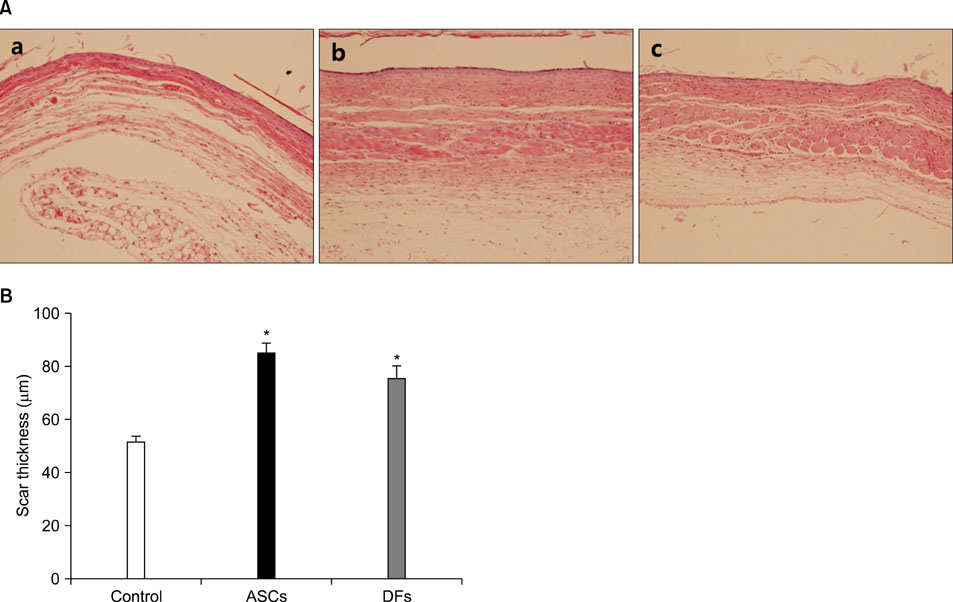
15-mm round full-thickness skin defects were generated on the back of BALB/c nude mice. The mice were divided into three groups for wound coverage: (i) human ASCs-populated collagen gel, (ii) human dermal fibroblasts-populated collagen gel, and (iii) collagen gel alone. Wound contraction was prevented with a splint method. Wound size was measured 10 days after injury. At 28 days histological analysis was performed.
RESULTS
Both ASCs and dermal fibroblasts accelerated wound closure, but dermal fibroblasts were more effective than ASCs. At 28 days, the dermal portion of ASCs or dermal fibroblasts wound scars were thicker than collagen gel wound scars.
CONCLUSION
ASCs and dermal fibroblasts stimulate cutaneous wound healing and improve scar thickness.
Keyword
MeSH Terms
Figure
Reference
-
1. Steeper R. A critical review of the aetiology of diabetic neuropathic ulcers. J Wound Care. 2005. 14:101–103.
Article2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article3. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001. 282:148–152.
Article4. Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001. 37:1726–1732.
Article5. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007. 25:2648–2659.
Article6. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008. 3:e1886.
Article7. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
Article8. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006. 99:1285–1297.
Article9. Nambu M, Kishimoto S, Nakamura S, Mizuno H, Yanagibayashi S, Yamamoto N, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009. 62:317–321.
Article10. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007. 48:15–24.
Article11. Park JS, Park WY, Cho KA, Kim DI, Jhun BH, Kim SR, et al. Down-regulation of amphiphysin-1 is responsible for reduced receptor-mediated endocytosis in the senescent cells. FASEB J. 2001. 15:1625–1627.
Article12. Galiano RD, Michaels J 5th, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004. 12:485–492.
Article13. Marks MG, Doillon C, Silver FH. Effects of fibroblasts and basic fibroblast growth factor on facilitation of dermal wound healing by type I collagen matrices. J Biomed Mater Res. 1991. 25:683–696.
Article14. Okamoto E, Kitano Y. Expression of basement membrane components in skin equivalents--influence of dermal fibroblasts. J Dermatol Sci. 1993. 5:81–88.
Article15. Maruguchi T, Maruguchi Y, Suzuki S, Matsuda K, Toda K, Isshiki N. A new skin equivalent: keratinocytes proliferated and differentiated on collagen sponge containing fibroblasts. Plast Reconstr Surg. 1994. 93:537–546.16. Medalie DA, Eming SA, Collins ME, Tompkins RG, Yarmush ML, Morgan JR. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. Transplantation. 1997. 64:454–465.
Article17. Lamme EN, van Leeuwen RT, Mekkes JR, Middelkoop E. Allogeneic fibroblasts in dermal substitutes induce inflammation and scar formation. Wound Repair Regen. 2002. 10:152–160.
Article18. Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006. 17:279–290.
Article19. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009. 17:540–547.
Article20. Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, et al. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008. 26:1275–1287.
Article21. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007. 110:3499–3506.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acceleration of Wound Healing Using Adipose-derived Stem Cell Therapy with Platelet Concentrates: Platelet-rich Plasma (PRP) vs. Platelet-rich Fibrin (PRF)
- Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing
- Effects of Adipose-derived Stromal Cells and of their Extract on Wound Healing in a Mouse Model
- Paracrine Effects of Adipose-Derived Stem Cells on Keratinocytes and Dermal Fibroblasts
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review