J Korean Med Sci.
2008 Apr;23(2):288-295. 10.3346/jkms.2008.23.2.288.
Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) Augments Acute Lung Injury via Its Neutrophil Priming Effects
- Affiliations
-
- 1Department of Internal Medicine, Chung Ang University College of Medicine, Seoul, Korea. jykimmd@cau.ac.kr
- KMID: 1713463
- DOI: http://doi.org/10.3346/jkms.2008.23.2.288
Abstract
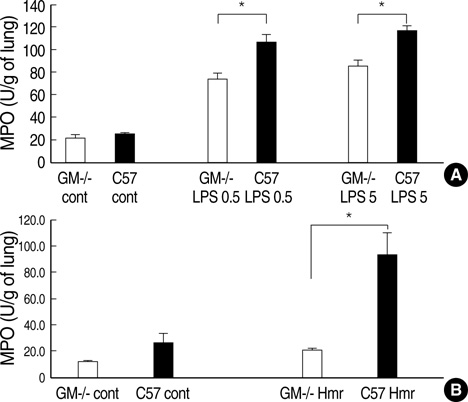
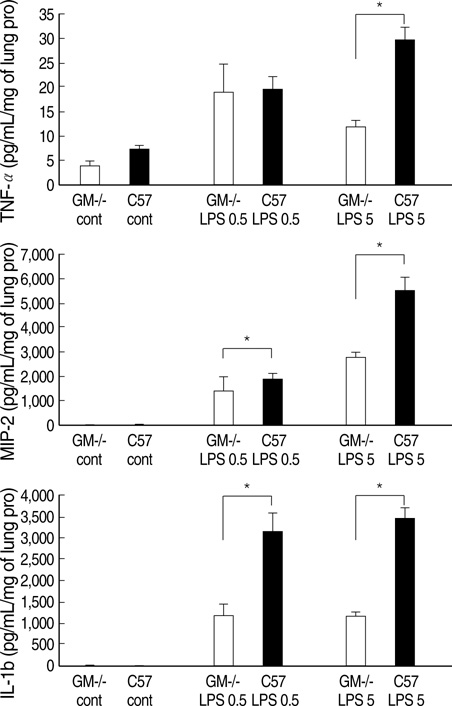
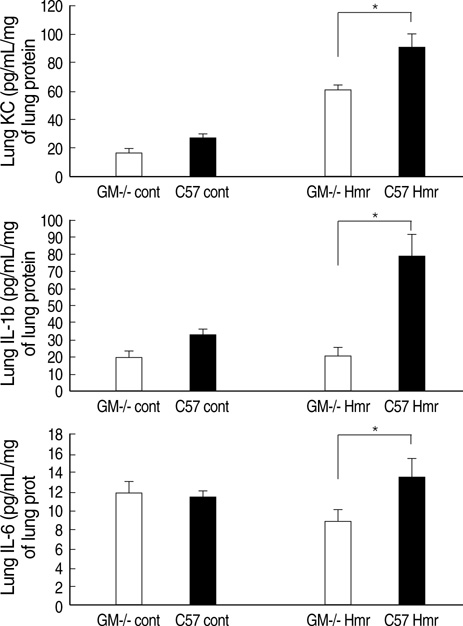
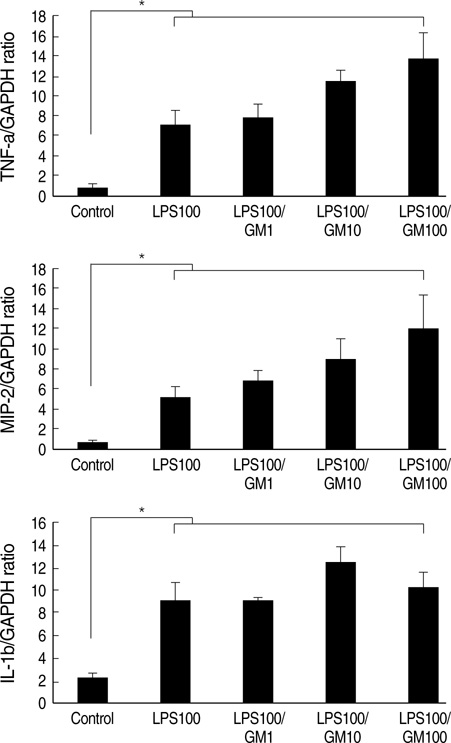
- Granulocyte macrophage-colony stimulating factor (GM-CSF) has immuno-stimulatory effects. We hypothesized that GM-CSF plays an important role both in lipopolysaccharide (LPS)- and hemorrhage-induced acute lung injury (ALI). We also postulated that GM-CSF augments LPS-induced inflammation by priming neutrophils. ALI was induced in GM-CSF-/- or control C57BL mice either by LPS injection or by hemorrhage. Lung inflammation (by lung expression for tumor necrosis factor-alpha (TNF-alpha), macrophage inflammatory protein-2 (MIP-2), interleukin-1beta (IL-1beta), interleukin- 6 (IL-6), and keratinocyte-derived chemokine) and lung injury (by myeloperoxidase and evans blue dye assay) were evaluated after ALI. Incremental doses of LPS (0, 1, 10, and 100 ng/mL) and GM-CSF (0, 1, 10, and 100 ng/mL) were added to bone marrow neutrophils. The expression of TNF-alpha, MIP-2, and IL-1beta was evaluated with enzyme linked immunosorbent assay. The mRNA expression of three cytokines, and the nuclear translocation of nuclear factor kappa B (NF kappa-B) were evaluated by reverse transcriptase-polymerase chain reaction and electropnoretic mobility shift assay, respectively. GM-CSF -/- mice showed decreased neutrophil infiltration, less leakage, and lower expression of cytokines in the lung after LPS or hemorrhage. GM-CSF augmented LPS-induced protein and mRNA expression of TNF-alpha, MIP-2 and IL-1beta, which was mediated by increased intra-nuclear translocation of NF-kappa B. GM-CSF plays an important role in high-dose LPS and hemorrhage-induced ALI, which appears to be mediated by its priming effect on neutrophils.
Keyword
MeSH Terms
-
Animals
Bone Marrow Cells/cytology
Chemokine CXCL2/metabolism
Granulocyte-Macrophage Colony-Stimulating Factor/metabolism/*physiology
Interleukin-1beta/metabolism
Lipopolysaccharides/metabolism
Lung/metabolism/pathology
*Lung Injury
Male
Mice
Mice, Inbred BALB C
Mice, Inbred C57BL
Mice, Transgenic
Neutrophils/*cytology/metabolism
Peroxidase/metabolism
Tumor Necrosis Factor-alpha/metabolism
Figure
Cited by 1 articles
-
The Role of Keratinocyte-derived Chemokine in Hemorrhage-induced Acute Lung Injury in Mice
Byoung Hoon Lee, Tae Jin Lee, Jae Woo Jung, Dong Jin Oh, Jae Chol Choi, Jong Wook Shin, In Won Park, Byoung Whui Choi, Jae Yeol Kim
J Korean Med Sci. 2009;24(5):775-781. doi: 10.3346/jkms.2009.24.5.775.
Reference
-
1. Fibbe WC, Daha MR, Hiemstra PS, Duinkerken N, Lurvink E, Ralph P, Altrock BW, Kaushansky K, Willemze R, Falkenburg JH. Interleukin1 and poly(rl)poly(rC) induce production of granulocyt CSF, macrophage CSF and granulocyte-macrophage CSF by human endothelial cells. Exp Hematol. 1989. 17:229–234.2. Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, Marks RM. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989. 243:1467–1469.
Article3. Gasson JC, Weisbart RH, Kaufman SE, Clark SC, Hewick RM, Wong GG, Golde DW. Purified human granulocyte-macrophage colony-stimulating factor: direct effects on neutrophils. Science. 1984. 226:1339–1342.4. Weisbart RH, Golde DW, Clark SC, Wong GG, Gasson JC. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985. 314:361–363.
Article5. Arnberg H, Letocha H, Nou F, Westlin JF, Nilsson S. GM-CSF in chemotherapy-induced febrile neutropenia--a double-blind randomized study. Anticancer Res. 1998. 18:1255–1260.6. Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, Waltersdorph A, Wong G, Clark SC, Vadas MA. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986. 78:1220–1228.
Article7. Verhoef G, Boogaerts M. Treatment with granulocyte-macrophage colony-stimulating factor and the adult respiratory distress syndrome. Am J Hematol. 1991. 36:285–287.8. Tiegs G, Barsig J, Matiba B, Uhlig S, Wendel A. Potentiation by granulocyte macrophage colony-stimulating factor of lipopolysaccharide toxicity in mice. J Clin Invest. 1994. 93:2616–2622.
Article9. Sauaia A, Moore FA, Moore EE, Lezotte DC. Early risk factors for postinjury multiple organ failure. World J Surg. 1996. 20:392–400.
Article10. Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994. 264:713–716.
Article11. Kim JY, Park JS, Strassheim S, Douglas I, del Valle FD, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005. 288:L958–L965.
Article12. Pelaez A, Bechara RI, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2004. 286:L106–L111.
Article13. Orozco H, Arch J, Medina-Franco H, Pantoja JP, Gonzalez QH, Vilatoba M, Hinojosa C, Vargas-Vorackova F, Sifuentes-Osornio J. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg. 2006. 141:150–153.14. Basu S, Dunn AR, Marino MW, Savoia H, Hodgson G, Lieschke GJ, Cebon J. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1997. 159:1412–1417.15. Kamiya K, Kono T, Iwamoto J, Yoneda M, Kotani H, Kasai S. The cytoprotective role of lipopolysaccharide-induced nitric oxide against liver damage during early phase of endotoxemia in rats. Shock. 2000. 14:229–233.
Article16. LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest. 1999. 103:563–569.
Article17. Abraham E, Nick JA, Azam T, Kim SH, Mira JP, Svetkauskaite D, He Q, Zamora M, Murphy J, Park JS, Overdier K, Dinarello CA. Peripheral blood neutrophil activation patterns are associated with pulmonary inflammatory responses to lipopolysaccharide in humans. J Immunol. 2006. 176:7753–7760.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma G-CSF and GM-CSF Concentrations and Expression of their Receptors on the Granulocyte in Children with Leukocytosis
- Plasma G-CSF and GM-CSF Concentration and Amount of Their Receptors on the Granulocyte in Kawasaki Disease
- A Case of Pulmonary Alveolar Proteinosis that Improved with GM-CSF Inhalation Therapy
- Effect of GM-CSF to the M-VAC Chemotherapy Induced Leukopenia in Patients with Urothelial Cancer
- A study of the effects of GM-CSF incubation on long-term human bone marrow cultures