Anat Cell Biol.
2010 Dec;43(4):294-302. 10.5115/acb.2010.43.4.294.
Mega-dose vitamin C attenuated lung inflammation in mouse asthma model
- Affiliations
-
- 1Department of Anatomy and Tumor Immunity Medical Research Center, Seoul National University College of Medicine, Seoul, Korea. hyi830@snu.ac.kr
- KMID: 1455343
- DOI: http://doi.org/10.5115/acb.2010.43.4.294
Abstract
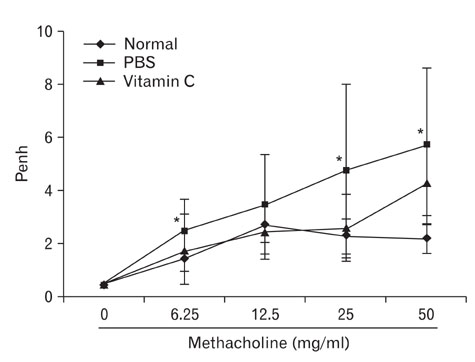
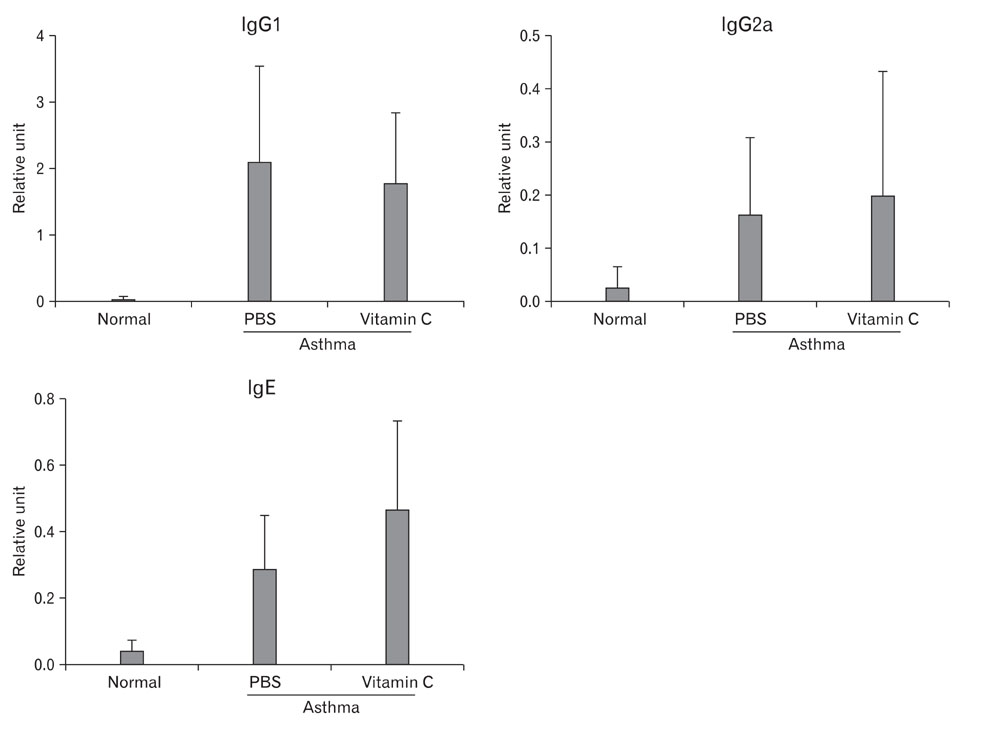
- Asthma is a Th2-dependent disease mediated by IgE and Th2 cytokines, and asthmatic patients suffer from oxidative stresses from abnormal airway inflammation. Vitamin C is a micro-nutrient functioning as an antioxidant. When administered at a mega-dose, vitamin C has been reported to shift immune responses toward Th1. Thus, we tried to determine whether vitamin C exerted beneficial effects in asthma animal model. Asthma was induced in mice by sensitizing and challenging with ovalbumin. At the time of challenge, 3~5 mg of vitamin C was administered and the effects were evaluated. Vitamin C did not modulate Th1/Th2 balance in asthma model. However, it decreased airway hyperreactivity to methacholine, decreased inflammatory cell numbers in brochoalveolar lavage fluid, and moderate reduction of perivascular and peribronchiolar inflammatory cell infiltration. These results suggest that vitamin C administered at the time of antigen challenge exerted anti-inflammatory effects. Further studies based on chronic asthma model are needed to evaluate a long-term effect of vitamin C in asthma. In conclusion, even though vitamin C did not show any Th1/Th2 shifting effects in this experiment, it still exerted moderate anti-inflammatory effects. Considering other beneficial effects and inexpensiveness of vitamin C, mega-dose usage of vitamin C could be a potential supplementary modality for the management of asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch Pathol Lab Med. 2006. 130:447–451.2. Bjermer L, Diamant Z. Current and emerging nonsteroidal anti-inflammatory therapies targeting specific mechanisms in asthma and allergy. Treat Respir Med. 2004. 3:235–246.3. Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008. 121:560–570.4. Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Golde DW. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry. 2002. 41:12995–13002.5. Chanez P, Wenzel SE, Anderson GP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007. 119:1337–1348.6. Chang HH, Chen CS, Lin JY. High dose vitamin C supplementation increases the Th1/Th2 cytokine secretion ratio, but decreases eosinophilic infiltration in bronchoalveolar lavage fluid of ovalbumin-sensitized and challenged mice. J Agric Food Chem. 2009. 57:10471–10476.7. Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol. 1998. 161:3813–3816.8. Desai D, Brightling C. Cytokine and anti-cytokine therapy in asthma: ready for the clinic? Clin Exp Immunol. 2009. 158:10–19.9. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007. 19:676–680.10. Du WD, Yuan ZR, Sun J, et al. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003. 9:2565–2569.11. Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004. 172:7053–7059.12. Fogarty A, Lewis SA, Scrivener SL, et al. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003. 33:1355–1359.13. Fogarty A, Lewis SA, Scrivener SL, et al. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respir Med. 2006. 100:174–179.14. Gao J, Gao X, Li W, Zhu Y, Thompson PJ. Observational studies on the effect of dietary antioxidants on asthma: a meta-analysis. Respirology. 2008. 13:528–536.15. Guaiquil VH, Vera JC, Golde DW. Mechanism of vitamin C inhibition of cell death induced by oxidative stress in glutathione-depleted HL-60 cells. J Biol Chem. 2001. 276:40955–40961.16. Haines DD, Varga B, Bak I, et al. Summative interaction between astaxanthin, Ginkgo biloba extract (EGb761) and vitamin C in Suppression of respiratory inflammation: a comparison with ibuprofen. Phytother Res. 2010. [Epub ahead of Print].17. Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009. 71:489–507.18. Harik-Khan RI, Muller DC, Wise RA. Serum vitamin levels and the risk of asthma in children. Am J Epidemiol. 2004. 159:351–357.19. Hernandez M, Zhou H, Zhou B, et al. Combination treatment with high-dose vitamin C and alpha-tocopherol does not enhance respiratory-tract lining fluid vitamin C levels in asthmatics. Inhal Toxicol. 2009. 21:173–181.20. Holgate ST, Holloway J, Wilson S, et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006. 117:496–506.21. Izuhara K, Ohta S, Shiraishi H, et al. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009. 16:2867–2875.22. Kamgar M, Zaldivar F, Vaziri ND, Pahl MV. Antioxidant therapy does not ameliorate oxidative stress and inflammation in patients with end-stage renal disease. J Natl Med Assoc. 2009. 101:336–344.23. Kaur B, Rowe BH, Arnold E. Vitamin C supplementation for asthma. Cochrane Database Syst Rev. 2009. (1):CD000993.24. Kelly FJ, Mudway I, Blomberg A, Frew A, Sandström T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999. 354:482–483.25. Kirby AC, Yrlid U, Svensson M, Wick MJ. Differential involvement of dendritic cell subsets during acute Salmonella infection. J Immunol. 2001. 166:6802–6811.26. Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001. 103:1863–1868.27. Lee CW, Wang XD, Chien KL, et al. Vitamin C supplementation does not protect L-gulono-gamma-lactone oxidase-deficient mice from Helicobacter pylori-induced gastritis and gastric premalignancy. Int J Cancer. 2008. 122:1068–1076.28. Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000. 97:841–846.29. Marleau AM, Summers KL, Singh B. Differential contributions of APC subsets to T cell activation in nonobese diabetic mice. J Immunol. 2008. 180:5235–5249.30. McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004. 34:497–507.31. Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003. 2:7.32. Nelde A, Teufel M, Hahn C, et al. The impact of the route and frequency of antigen exposure on the IgE response in allergy. Int Arch Allergy Immunol. 2001. 124:461–469.33. Noh K, Kim HG, Shin YA, et al. The effects of high-dose vitamin C administration on the cell-mediated immune response in mice. Immune Netw. 2003. 3:211–218.34. Noh K, Lim H, Moon SK, et al. Mega-dose Vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol Lett. 2005. 98:63–72.35. Rizzo MR, Abbatecola AM, Barbieri M, et al. Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: impact on insulin action. J Am Coll Nutr. 2008. 27:505–511.36. Sackesen C, Ercan H, Dizdar E, et al. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol. 2008. 122:78–85.37. Schock BC, Young IS, Brown V, Fitch PS, Shields MD, Ennis M. Antioxidants and oxidative stress in BAL fluid of atopic asthmatic children. Pediatr Res. 2003. 53:375–381.38. Scott DA, Poston RN, Wilson RF, Coward PY, Palmer RM. The influence of vitamin C on systemic markers of endothelial and inflammatory cell activation in smokers and non-smokers. Inflamm Res. 2005. 54:138–144.39. Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int. 2007. 56:331–340.40. Tecklenburg SL, Mickleborough TD, Fly AD, Bai Y, Stager JM. Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir Med. 2007. 101:1770–1778.41. Tulic MK, Hamid Q. New insights into the pathophysiology of the small airways in asthma. Clin Chest Med. 2006. 27:41–52.42. Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. 2006. 83:567–574.43. Zhou J, Wolf CR, Henderson CJ, et al. Glutathione transferase P1: an endogenous inhibitor of allergic responses in a mouse model of asthma. Am J Respir Crit Care Med. 2008. 178:1202–1210.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intranasal Administration of Unmethylated CpG with Cockroach Antigen Prevents the Development of Cockroach-Induced Allergic Inflammation
- Understanding asthma using animal models
- Trends of vitamin D in asthma in the pediatric population for two decades: a systematic review
- The Preventive Effect of Allergic Inflammation by Induction of Oral Tolerance in a Mouse Model of Chronic Asthma
- Effect of Cytosine-phosphate-Guanine Oligodeoxynucleotides on Airway Hyperresponsiveness and Inflammation of Repeated Respiratory Syncytial Virus Infection in a Mouse Model