Anat Cell Biol.
2011 Jun;44(2):106-115. 10.5115/acb.2011.44.2.106.
Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebral cortex and in the hippocampal region of nNOS knock-out(-/-) mice
- Affiliations
-
- 1Department of Anatomy, College of Medicine, Seoul National University, Seoul, Korea. kmjoo@snu.ac.kr
- 2Department of Neurosurgery, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- KMID: 1447420
- DOI: http://doi.org/10.5115/acb.2011.44.2.106
Abstract
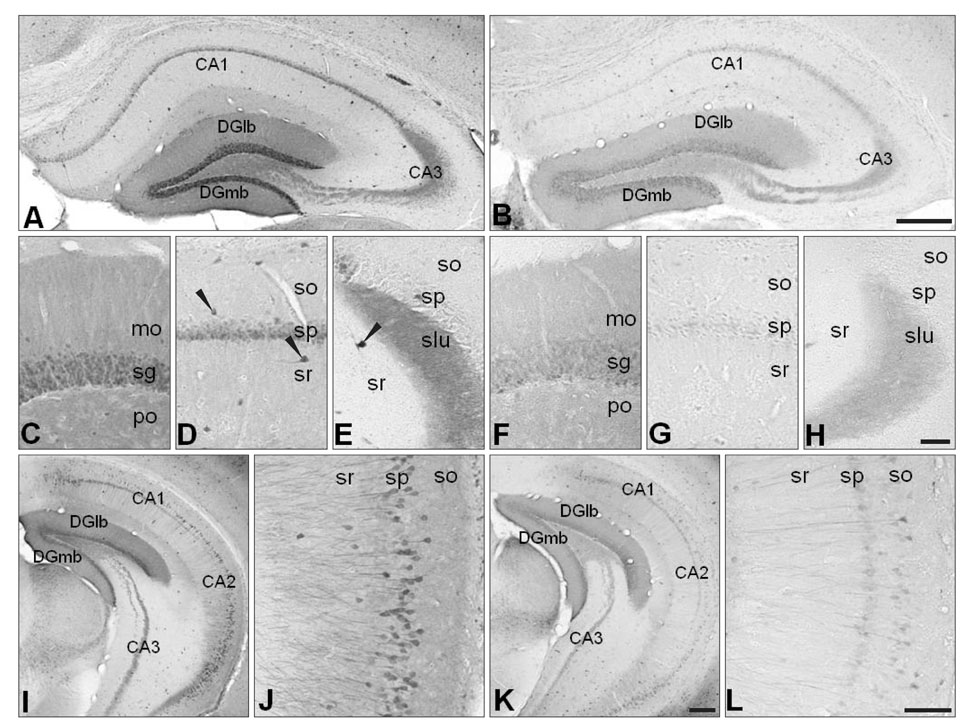
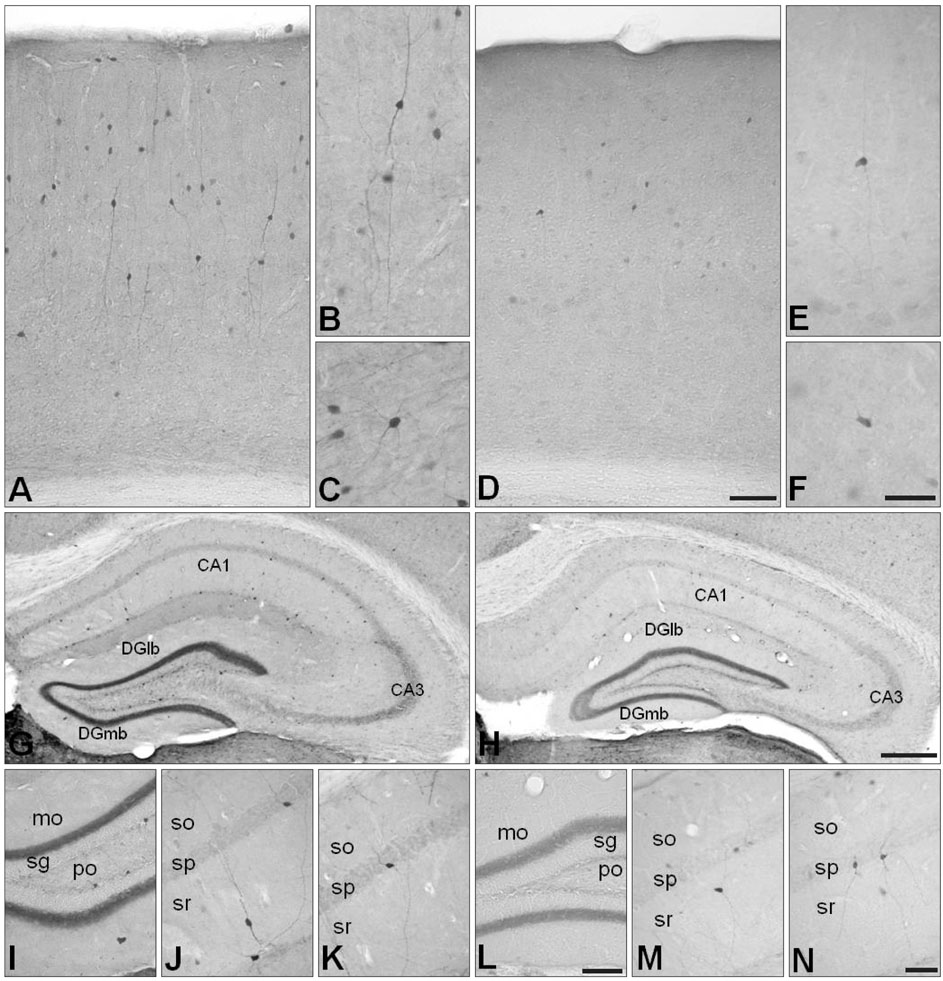
- Nitric oxide (NO) modulates the activities of various channels and receptors to participate in the regulation of neuronal intracellular Ca2+ levels. Ca2+ binding protein (CaBP) expression may also be altered by NO. Accordingly, we examined expression changes in calbindin-D28k, calretinin, and parvalbumin in the cerebral cortex and hippocampal region of neuronal NO synthase knockout(-/-) (nNOS-/-) mice using immunohistochemistry. For the first time, we demonstrate that the expression of CaBPs is specifically altered in the cerebral cortex and hippocampal region of nNOS-/- mice and that their expression changed according to neuronal type. As changes in CaBP expression can influence temporal and spatial intracellular Ca2+ levels, it appears that NO may be involved in various functions, such as modulating neuronal Ca2+ homeostasis, regulating synaptic transmission, and neuroprotection, by influencing the expression of CaBPs. Therefore, these results suggest another mechanism by which NO participates in the regulation of neuronal Ca2+ homeostasis. However, the exact mechanisms of this regulation and its functional significance require further investigation.
Keyword
MeSH Terms
-
Animals
Calcium
Calcium-Binding Protein, Vitamin D-Dependent
Calcium-Binding Proteins
Carrier Proteins
Cerebral Cortex
Homeostasis
Immunohistochemistry
Mice
Neurons
Nitric Oxide
Nitric Oxide Synthase
Synaptic Transmission
Calcium
Calcium-Binding Protein, Vitamin D-Dependent
Calcium-Binding Proteins
Carrier Proteins
Nitric Oxide
Nitric Oxide Synthase
Figure
Reference
-
1. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993. 329:2002–2012.2. Liu PK, Robertson CS, Valadka A. The association between neuronal nitric oxide synthase and neuronal sensitivity in the brain aft er brain injury. Ann N Y Acad Sci. 2002. 962:226–241.3. Clementi E, Riccio M, Sciorati C, Nisticò G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. Role of the nitric oxide/cGMP pathway. J Biol Chem. 1996. 271:17739–17745.4. Short DM, Heron ID, Birse-Archbold JL, Kerr LE, Sharkey J, McCulloch J. Apoptosis induced by staurosporine alters chaperone and endoplasmic reticulum proteins: identification by quantitative proteomics. Proteomics. 2007. 7:3085–3096.5. Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000. 1:120–129.6. Rogers JH. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol. 1987. 105:1343–1353.7. Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci. 1996. 19:547–554.8. Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996. 21:134–140.9. Geula C, Schatz CR, Mesulam MM. Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience. 1993. 54:461–476.10. Bertini G, Peng ZC, Bentivoglio M. The chemical heterogeneity of cortical interneurons: nitric oxide synthase vs. calbindin and parvalbumin immunoreactivity in the rat. Brain Res Bull. 1996. 39:261–266.11. Arévalo R, Sánchez F, Alonso JR, Rubio M, Aijón J, Vázquez R. Infrequent cellular coexistence of NADPH-diaphorase and calretinin in the neurosecretory nuclei and adjacent areas of the rat hypothalamus. J Chem Neuroanat. 1993. 6:335–341.12. Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995. 378:383–386.13. Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci U S A. 1997. 94:1488–1493.14. Cheron G, Schurmans S, Lohof A, d'Alcantara P, Meyer M, Draye JP, Parmentier M, Schiffmann SN. Electrophysiological behavior of Purkinje cells and motor coordination in calretinin knock-out mice. Prog Brain Res. 2000. 124:299–308.15. Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993. 75:1273–1286.16. Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992. 15:303–308.17. Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996. 6:347–470.18. Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997. 7:476–486.19. Lee JC, Cho YJ, Kim J, Kim N, Kang BG, Cha CI, Joo KM. Region-specific changes in the immunoreactivity of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC2, and PAC1 receptor) in the aged rat brains. Brain Res. 2010. 1351:32–40.20. DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997. 14:1–19.21. Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and chole cy stokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002. 31:277–287.22. Jinno S, Kosaka T. Patterns of expression of calcium binding proteins and neuronal nitric oxide synthase in different populations of hippocampal GABAergic neurons in mice. J Comp Neurol. 2002. 449:1–25.23. Jouvenceau A, Potier B, Battini R, Ferrari S, Dutar P, Billard JM. Glutamatergic synaptic responses and long-term potentiation are impaired in the CA1 hippocampal area of calbindin D(28k)-deficient mice. Synapse. 1999. 33:172–180.24. Schurmans S, Schiffmann SN, Gurden H, Lemaire M, Lipp HP, Schwam V, Pochet R, Imperato A, Böhme GA, Parmentier M. Impaired long-term potentiation induction in dentate gyrus of calretinin-deficient mice. Proc Natl Acad Sci U S A. 1997. 94:10415–10420.25. Parra P, Gulyás AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998. 20:983–993.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebellum of the nNOS knock-out(-/-) mice
- Seizure -Related Change of NADPH -diaphorase and Calcium Binding Protein Positive Neurons in the Brain of Rats
- The distribution of calbindin-D28k, parvalbumin, and calretinin immunoreactivity in the inferior colliculus of circling mouse
- Immunocytochemical Studies of Calbindin D-28k and Parvalbumin in the Sensory and motor Cortex of the Cat
- Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina