Anat Cell Biol.
2017 Sep;50(3):230-238. 10.5115/acb.2017.50.3.230.
The distribution of calbindin-D28k, parvalbumin, and calretinin immunoreactivity in the inferior colliculus of circling mouse
- Affiliations
-
- 1Department of Pharmacology, Dankook University College of Medicine, Cheonan, Korea.
- 2Department of Anatomy, Dankook University College of Medicine, Cheonan, Korea. mjukim99@dankook.ac.kr
- KMID: 2390485
- DOI: http://doi.org/10.5115/acb.2017.50.3.230
Abstract
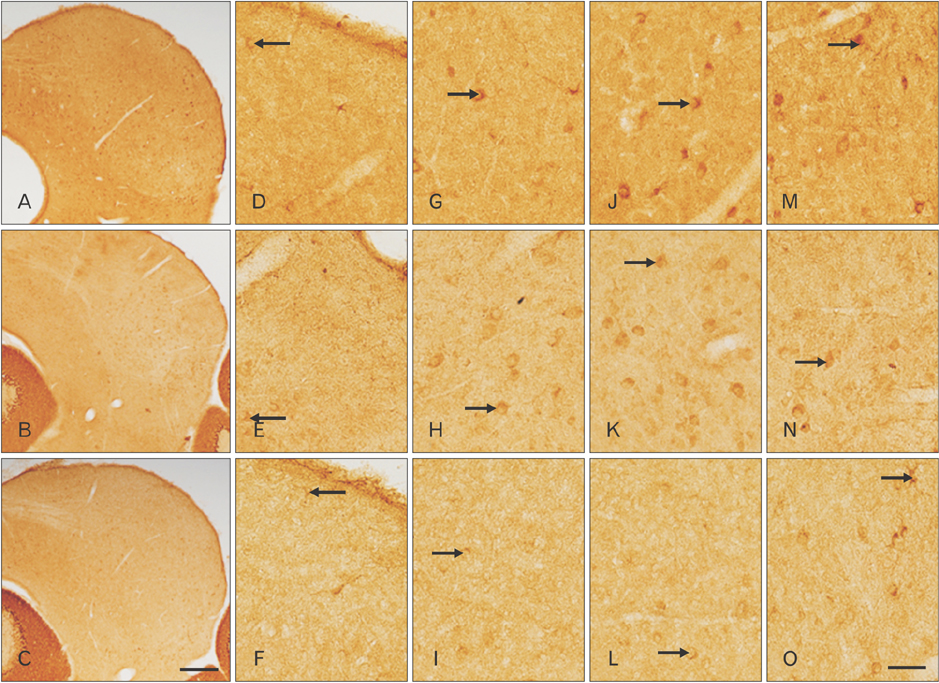
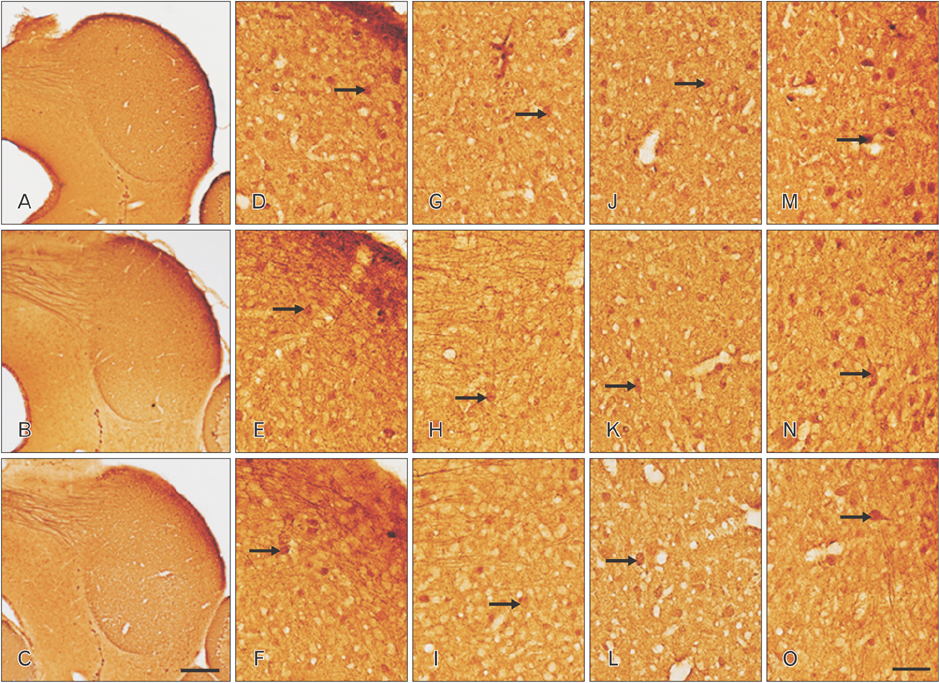
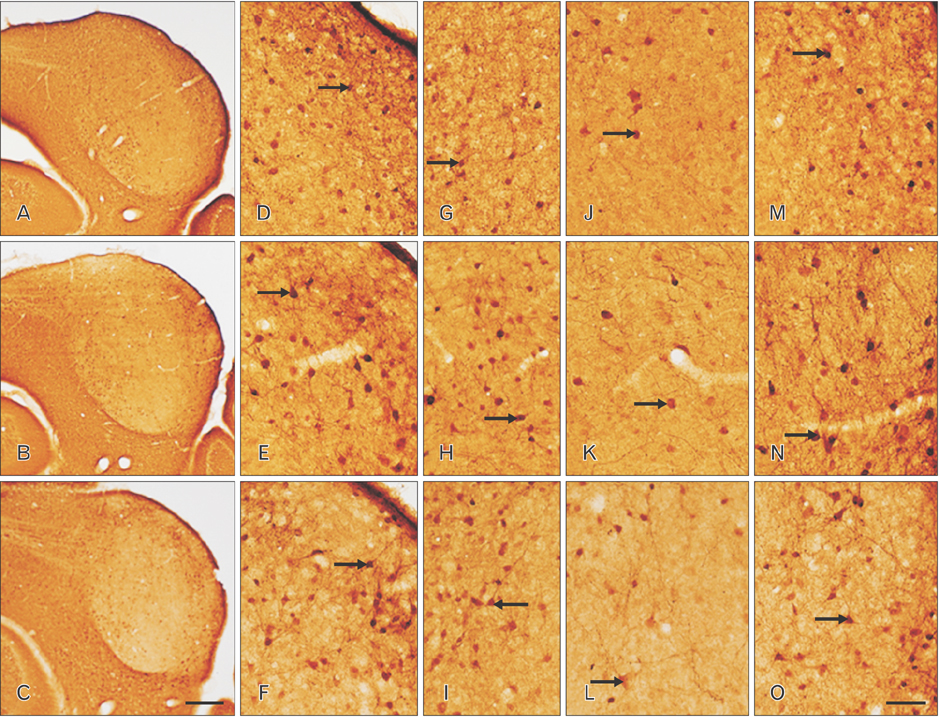
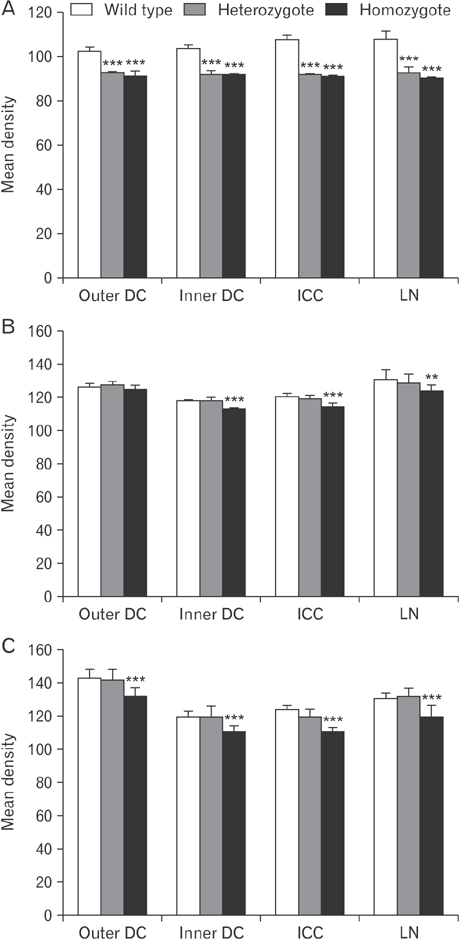
- The circling mice with tmie gene mutation are known as an animal deafness model, which showed hyperactive circling movement. Recently, the reinvestigation of circling mouse was performed to check the inner ear pathology as a main lesion of early hearing loss. In this trial, the inner ear organs were not so damaged to cause the hearing deficit of circling (cir/cir) mouse at 18 postnatal day (P18) though auditory brainstem response data indicated hearing loss of cir/cir mice at P18. Thus, another mechanism may be correlated with the early hearing loss of cir/cir mice at P18. Hearing loss in the early life can disrupt the ascending and descending information to inferior colliculus (IC) as integration site. There were many reports that hearing loss could result in the changes in Ca²âº concentration by either cochlear ablation or genetic defect. However, little was known to be reported about the correlation between the pathology of IC and Ca²âº changes in circling mice. Therefore, the present study investigated the distribution of calcium-binding proteins (CaBPs), calbindin-D28k, parvalbumin, and calretinin immunoreactivity (IR) in the IC to compare among wild-type (+/+), heterozygous (+/cir), and homozygous (cir/cir) mice by immunohistochemistry. The decreases of CaBPs IR in cir/cir were statistically significant in the neurons as well as neuropil of IC. Thus, this study proposed overall distributional alteration of CaBPs IR in the IC caused by early hearing defect and might be helpful to elucidate the pathology of central auditory disorder related with Ca²âº metabolism.
MeSH Terms
Figure
Reference
-
1. Shin MJ, Lee JH, Yu DH, Kim BS, Kim HJ, Kim SH, Kim MO, Park C, Hyun BH, Lee S, So HS, Park R, Ryoo ZY. Ectopic expression of tmie transgene induces various recovery levels of behavior and hearing ability in the circling mouse. Biochem Biophys Res Commun. 2008; 374:17–21.2. Cho KI, Lee JW, Kim KS, Lee EJ, Suh JG, Lee HJ, Kim HT, Hong SH, Chung WH, Chang KT, Hyun BH, Oh YS, Ryoo ZY. Fine mapping of the circling (cir) gene on the distal portion of mouse chromosome 9. Comp Med. 2003; 53:642–648.3. Chung WH, Kim KR, Cho YS, Cho DY, Woo JH, Ryoo ZY, Cho KI, Hong SH. Cochlear pathology of the circling mouse: a new mouse model of DFNB6. Acta Otolaryngol. 2007; 127:244–251.4. Lee Y, Chang SY, Jung JY, Ahn SC. Reinvestigation of cochlear pathology in circling mice. Neurosci Lett. 2015; 594:30–35.5. Illing RB. Activity-dependent plasticity in the adult auditory brainstem. Audiol Neurootol. 2001; 6:319–345.6. Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002; 82:601–636.7. Franklin SR, Brunso-Bechtold JK, Henkel CK. Unilateral cochlear ablation before hearing onset disrupts the maintenance of dorsal nucleus of the lateral lemniscus projection patterns in the rat inferior colliculus. Neuroscience. 2006; 143:105–115.8. Franklin SR, Brunso-Bechtold JK, Henkel CK. Bilateral cochlear ablation in postnatal rat disrupts development of banded pattern of projections from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. Neuroscience. 2008; 154:346–354.9. Ito T, Hirose J, Murase K, Ikeda H. Determining auditory-evoked activities from multiple cells in layer 1 of the dorsal cortex of the inferior colliculus of mice by in vivo calcium imaging. Brain Res. 2014; 1590:45–55.10. Ono M, Ito T. Functional organization of the mammalian auditory midbrain. J Physiol Sci. 2015; 65:499–506.11. Irvine DR. Physiology of the auditory brainstem. In : Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neurophysiology. New York: Springer;1992. p. 153–231.12. Oliver DL, Huerta MF. Inferior and superior colliculi. In : Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. New York: Springer;1992. p. 168–221.13. Patel MB, Sons S, Yudintsev G, Lesicko AM, Yang L, Taha GA, Pierce SM, Llano DA. Anatomical characterization of subcortical descending projections to the inferior colliculus in mouse. J Comp Neurol. 2017; 525:885–900.14. Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987; 266:519–534.15. Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988; 276:188–208.16. German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992; 648:42–62.17. Mammano F. Ca2+ homeostasis defects and hereditary hearing loss. Biofactors. 2011; 37:182–188.18. Tong B, Hornak AJ, Maison SF, Ohlemiller KK, Liberman MC, Simmons DD. Oncomodulin, an EF-hand Ca2+ Buffer, is critical for maintaining cochlear function in mice. J Neurosci. 2016; 36:1631–1635.19. Yew DT, Luo CB, Heizmann CW, Chan WY. Differential expression of calretinin, calbindin D28K and parvalbumin in the developing human cerebellum. Brain Res Dev Brain Res. 1997; 103:37–45.20. Maskey D, Pradhan J, Kim HJ, Park KS, Ahn SC, Kim MJ. Immunohistochemical localization of calbindin D28-k, parvalbumin, and calretinin in the cerebellar cortex of the circling mouse. Neurosci Lett. 2010; 483:132–136.21. Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. 2001. San Diego, CA: Academic Press;2001.22. Hoormann J, Falkenstein M, Hohnsbein J. Effect of selective attention on the latency of human frequency-following potentials. Neuroreport. 1994; 5:1609–1612.23. Idrizbegovic E, Bogdanovic N, Willott JF, Canlon B. Age-related increases in calcium-binding protein immunoreactivity in the cochlear nucleus of hearing impaired C57BL/6J mice. Neurobiol Aging. 2004; 25:1085–1093.24. Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990; 35:375–475.25. Friauf E. Distribution of calcium-binding protein calbindin-D28k in the auditory system of adult and developing rats. J Comp Neurol. 1994; 349:193–211.26. Rogers JH, Résibois A. Calretinin and calbindin-D28k in rat brain: patterns of partial co-localization. Neuroscience. 1992; 51:843–865.27. Zettel ML, Carr CE, O'Neill WE. Calbindin-like immunoreactivity in the central auditory system of the mustached bat, Pteronotus parnelli. J Comp Neurol. 1991; 313:1–16.28. Kelley PE, Frisina RD, Zettel ML, Walton JP. Differential calbindin-like immunoreactivity in the brain stem auditory system of the chinchilla. J Comp Neurol. 1992; 320:196–212.29. Zettel ML, Frisina RD, Haider SE, O'Neill WE. Age-related changes in calbindin D-28k and calretinin immunoreactivity in the inferior colliculus of CBA/CaJ and C57Bl/6 mice. J Comp Neurol. 1997; 386:92–110.30. Lohmann C, Friauf E. Distribution of the calcium-binding proteins parvalbumin and calretinin in the auditory brainstem of adult and developing rats. J Comp Neurol. 1996; 367:90–109.31. Tardif E, Chiry O, Probst A, Magistretti PJ, Clarke S. Patterns of calcium-binding proteins in human inferior colliculus: identification of subdivisions and evidence for putative parallel systems. Neuroscience. 2003; 116:1111–1121.32. Paloff AM, Usunoff KG, Yotovski P, Hinova-Palova DV, Ovtscharoff WA. Parvalbumin-like immunostaining in the cat inferior colliculus: light and electron microscopic investigation. Acta Histochem. 2004; 106:219–234.33. Idrizbegovic E, Bogdanovic N, Canlon B. Sound stimulation increases calcium-binding protein immunoreactivity in the inferior colliculus in mice. Neurosci Lett. 1999; 259:49–52.34. Vater M, Braun K. Parvalbumin, calbindin D-28k, and calretinin immunoreactivity in the ascending auditory pathway of horseshoe bats. J Comp Neurol. 1994; 341:534–558.35. Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc Natl Acad Sci U S A. 1996; 93:8005–8010.36. Ouda L, Druga R, Syka J. Changes in parvalbumin immunoreactivity with aging in the central auditory system of the rat. Exp Gerontol. 2008; 43:782–789.37. Zettel ML, O'Neill WE, Trang TT, Frisina RD. Early bilateral deafening prevents calretinin up-regulation in the dorsal cortex of the inferior colliculus of aged CBA/CaJ mice. Hear Res. 2001; 158:131–138.38. Hong SH, Kim MJ, Ahn SC. Glutamatergic transmission is sustained at a later period of development of medial nucleus of the trapezoid body-lateral superior olive synapses in circling mice. J Neurosci. 2008; 28:13003–13007.39. Willott JF, Bross LS, McFadden SL. Morphology of the inferior colliculus in C57BL/6J and CBA/J mice across the life span. Neurobiol Aging. 1994; 15:175–183.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebellum of the nNOS knock-out(-/-) mice
- Immunoreactivity of Calcium-Binding Proteins in the Central Auditory Nervous System of Aged Rats
- Postnatal Development of Parvalbumin and Calbindin D-28k Immunoreactivities in the Canine Anterior Cingulate Cortex: Transient Expression in Layer V Pyramidal Cells
- Immunocytochemical Study of Calcium Binding Protein in the Distal Nephron of Rat Kidney
- Calbindin-D28K Prevents Staurosporin-induced Bax Cleavage and Membrane Permeabilization