Anat Cell Biol.
2010 Sep;43(3):218-229. 10.5115/acb.2010.43.3.218.
Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina
- Affiliations
-
- 1Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul, Korea. ibkimmd@catholic.ac.kr
- KMID: 2168877
- DOI: http://doi.org/10.5115/acb.2010.43.3.218
Abstract
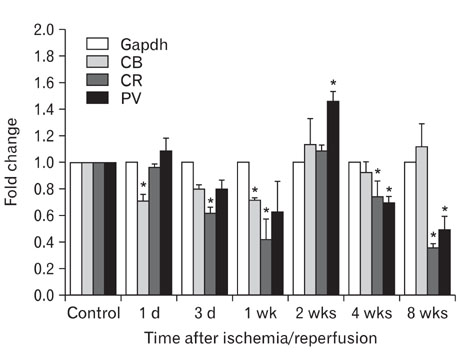
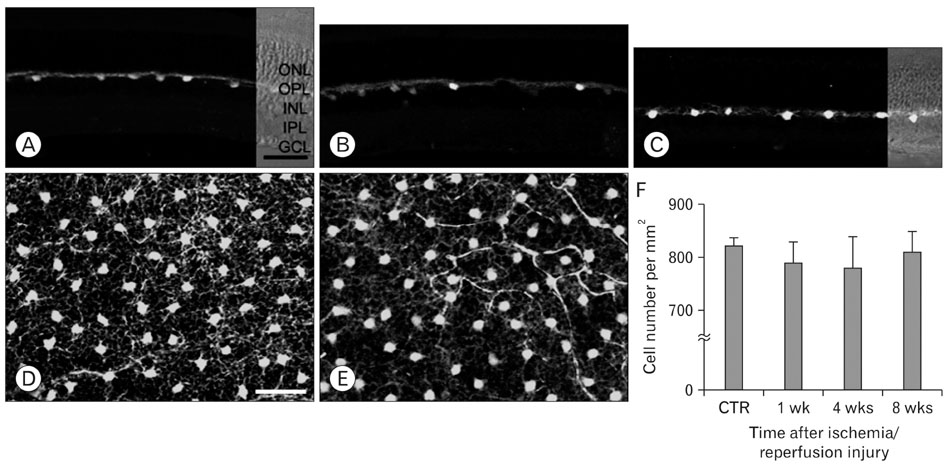
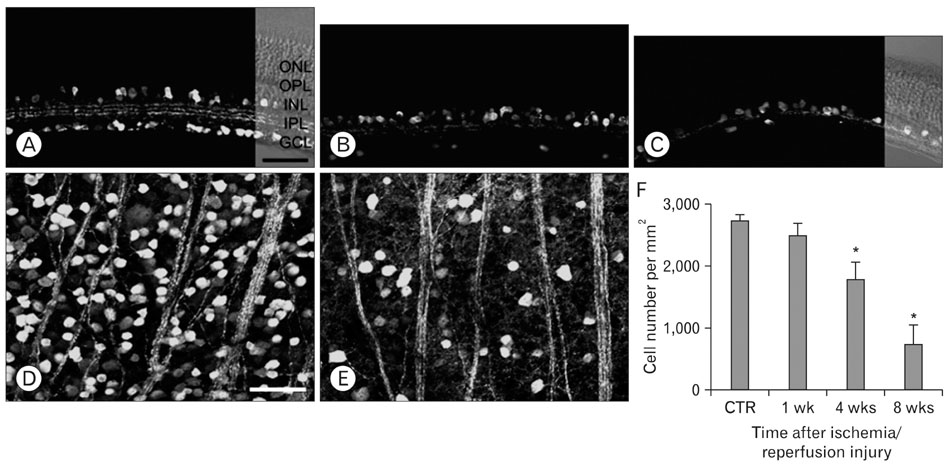
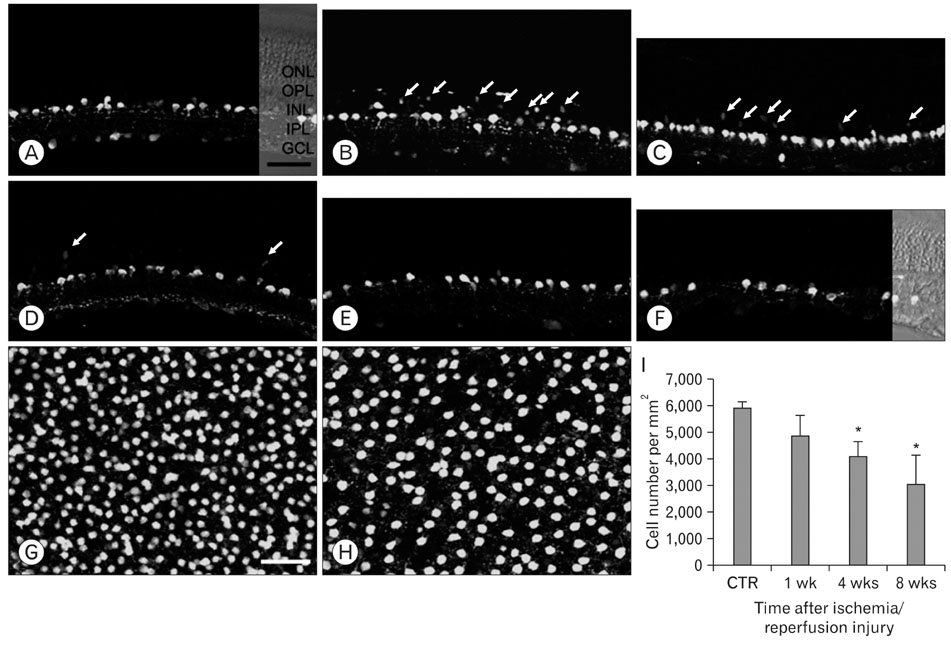
- Excessive calcium is thought to be a critical step in various neurodegenerative processes including ischemia. Calbindin D28k (CB), calretinin (CR), and parvalbumin (PV), members of the EF-hand calcium-binding protein family, are thought to play a neuroprotective role in various pathologic conditions by serving as a buffer against excessive calcium. The expression of CB, PV and CR in the ischemic rat retina induced by increasing intraocular pressure was investigated at the transcript and protein levels, by means of the quantitative real-time reverse transcription-polymerase chain reaction, western blot and immunohistochemistry. The transcript and protein levels of CB, which is strongly expressed in the horizontal cells in both normal and affected retinas, were not changed significantly and the number of CB-expressing horizontal cells remained unchanged throughout the experimental period 8 weeks after ischemia/reperfusion injury. At both the transcript and protein levels, however, CR, which is strongly expressed in several types of amacrine, ganglion, and displaced amacrine cells in both normal and affected retinas, was decreased. CR-expressing ganglion cell number was particularly decreased in ischemic retinas. Similar to the CR, PV transcript and protein levels, and PV-expressing AII amacrine cell number were decreased. Interestingly, in ischemic retinas PV was transiently expressed in putative cone bipolar cell types possibly those that connect with AII amacrine cells via gap junctions. These results suggest that these three calcium binding proteins may play different neuroprotective roles in ischemic insult by their ability to buffer calcium in the rat retina.
Keyword
MeSH Terms
-
Amacrine Cells
Animals
Blotting, Western
Calcium
Calcium-Binding Protein, Vitamin D-Dependent
Calcium-Binding Proteins
Cell Count
Ganglion Cysts
Gap Junctions
Humans
Immunohistochemistry
Intraocular Pressure
Ischemia
Neurons
Proteins
Rats
Retina
Calcium
Calcium-Binding Protein, Vitamin D-Dependent
Calcium-Binding Proteins
Proteins
Figure
Reference
-
1. Adachi K, Fujita Y, Morizane C, et al. Inhibition of NMDA receptors and nitric oxide synthase reduces ischemic injury of the retina. Eur J Pharmacol. 1998. 350:53–57.2. Ahmed BY, Toyoshima T, Yamagami S, et al. A chronological study of the expression of glial fibrillary acidic protein and calbindin-D28 k by reactive astrocytes in the electrically lesioned rat brain. Neurosci Res. 1996. 26:271–278.3. Azcona A, Lataste X. Isradipine in patients with acute ischaemic cerebral infarction. An overview of the ASCLEPIOS Programme. Drugs. 1990. 40:Suppl 2. 52–57.4. Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992. 15:303–308.5. Batini C, Guegan M, Palestini M, Thomasset M, Vigot R. Upregulation of Calbindin-D-28k immunoreactivity by excitatory amino acids. Arch Ital Biol. 1997. 135:385–397.6. Batini C, Palestini M, Thomasset M, Vigot R. Cytoplasmic calcium buffer, calbindin-D28k, is regulated by excitatory amino acids. Neuroreport. 1993. 4:927–930.7. Büchi ER. Cell death in rat retina after pressure-induced ischaemia-reperfusion insult: electron microscopic study. II. Outer nuclear layer. Jpn J Ophthalmol. 1992a. 36:62–68.8. Büchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992b. 55:605–613.9. Büchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991. 203:138–147.10. Celio MR, Baier W, Schärer L, Gregersen HJ, de Viragh PA, Norman AW. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium. 1990. 11:599–602.11. Choi DW. Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol. 1996. 6:667–672.12. Choi DW. Excitotoxicity, apoptosis and ischemic stroke. J Biochem Mol Biol. 2001. 34:8–14.13. Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993. 332:421–432.14. Chun MH, Kim IB, Ju WK, et al. Horizontal cells of the rat retina are resistant to degenerative processes induced by ischemia-reperfusion. Neurosci Lett. 1999. 260:125–128.15. D'Orlando C, Celio MR, Schwaller B. Calretinin and calbindin D-28k, but not parvalbumin protect against glutamate-induced delayed excitotoxicity in transfected N18-RE 105 neuroblastoma-retina hybrid cells. Brain Res. 2002. 945:181–190.16. D'Orlando C, Fellay B, Schwaller B, et al. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 2001. 909:145–158.17. Dijk F, Kamphuis W. An immunocytochemical study on specific amacrine cell subpopulations in the rat retina after ischemia. Brain Res. 2004. 1026:205–217.18. Dijk F, van Leeuwen S, Kamphuis W. Differential effects of ischemia/reperfusion on amacrine cell subtype-specific transcript levels in the rat retina. Brain Res. 2004. 1026:194–204.19. Fan Y, Shi L, Gu Y, et al. Pretreatment with PTD-calbindin D 28k alleviates rat brain injury induced by ischemia and reperfusion. J Cereb Blood Flow Metab. 2007. 27:719–728.20. Flagg-Newton J, Simpson I, Loewenstein WR. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979. 205:404–407.21. Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998. 95:3227–3232.22. Heizmann CW, Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992. 15:259–264.23. Hwang IK, Kang TC, Lee JC, et al. Chronological alterations of calbindin D-28k immunoreactivity in the gerbil main olfactory bulb after ischemic insult. Brain Res. 2003. 971:250–254.24. Joo CK, Choi JS, Ko HW, et al. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999. 40:713–720.25. Kageyama T, Ishikawa A, Tamai M. Glutamate elevation in rabbit vitreous during transient ischemia-reperfusion. Jpn J Ophthalmol. 2000. 44:110–114.26. Kim IB, Kim KY, Joo CK, et al. Reaction of Müller cells after increased intraocular pressure in the rat retina. Exp Brain Res. 1998. 121:419–424.27. Kim KY, Ju WK, Neufeld AH. Neuronal susceptibility to damage: comparison of the retinas of young, old and old/caloric restricted rats before and after transient ischemia. Neurobiol Aging. 2004. 25:491–500.28. Kim SK, Cho KO, Kim SY. White matter damage and hippocampal neurodegeneration induced by permanent bilateral occlusion of common carotid artery in the rat: comparison between wistar and sprague-dawley strain. Korean J Physiol Pharmacol. 2008. 12:89–94.29. Kristián T, Gidö G, Kuroda S, Schütz A, Siesjö BK. Calcium metabolism of focal and penumbral tissues in rats subjected to transient middle cerebral artery occlusion. Exp Brain Res. 1998. 120:503–509.30. Kwon OJ, Kim JY, Kim SY, Jeon CJ. Alterations in the localization of calbindin D28K-, calretinin-, and parvalbumin-immunoreactive neurons of rabbit retinal ganglion cell layer from ischemia and reperfusion. Mol Cells. 2005. 19:382–390.31. Lee GA, Lin CH, Jiang HH, Chao HJ, Wu CL, Hsueh CM. Microglia-derived glial cell line-derived neurotrophic factor could protect Sprague-Dawley rat astrocyte from in vitro ischemia-induced damage. Neurosci Lett. 2004. 356:111–114.32. Lee JC, Hwang IK, Yoo KY, et al. Calbindin D-28k is expressed in the microvascular basal lamina in the ventral horn at early time after transient spinal cord ischemia in the rabbit. Brain Res. 2005. 1047:123–128.33. Lewit-Bentley A, Réty S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000. 10:637–643.34. Li SY, Fu ZJ, Ma H, et al. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Invest Ophthalmol Vis Sci. 2009. 50:836–843.35. Limburg M, Hijdra A. Flunarizine in acute ischemic stroke: a pilot study. Eur Neurol. 1990. 30:121–122.36. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193:265–275.37. Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996. 366:15–33.38. Massey SC, Mills SL. Gap junctions between AII amacrine cells and calbindin-positive bipolar cells in the rabbit retina. Vis Neurosci. 1999. 16:1181–1189.39. Meier TJ, Ho DY, Park TS, Sapolsky RM. Gene transfer of calbindin D28k cDNA via herpes simplex virus amplicon vector decreases cytoplasmic calcium ion response and enhances neuronal survival following glutamatergic challenge but not following cyanide. J Neurochem. 1998. 71:1013–1023.40. Mojumder DK, Wensel TG, Frishman LJ. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol Vis. 2008. 14:1600–1613.41. Park HS, Park SJ, Park SH, Chun MH, Oh SJ. Shifting of parvalbumin expression in the rat retina in experimentally induced diabetes. Acta Neuropathol. 2008. 115:241–248.42. Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990. 5:1–16.43. Perlman JI, McCole SM, Pulluru P, Chang CJ, Lam TT, Tso MO. Disturbances in the distribution of neurotransmitters in the rat retina after ischemia. Curr Eye Res. 1996. 15:589–596.44. Peterson GL. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979. 100:201–220.45. Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci. 1996. 19:547–554.46. Rogers JH. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol. 1987. 105:1343–1353.47. Rosenbaum D, Zabramski J, Frey J, et al. Early treatment of ischemic stroke with a calcium antagonist. Stroke. 1991. 22:437–441.48. Sanna PP, Keyser KT, Battenberg E, Bloom FE. Parvalbumin immunoreactivity in the rat retina. Neurosci Lett. 1990. 118:136–139.49. Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996. 21:134–140.50. Toyoshima T, Yamagami S, Ahmed BY, et al. Expression of calbindin-D28K by reactive astrocytes in gerbil hippocampus after ischaemia. Neuroreport. 1996. 7:2087–2091.51. Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci. 1998. 18:10594–10602.52. Wässle H, Grünert U, Röhrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993. 332:407–420.53. White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000. 179:1–33.54. Xia XB, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci. 2004. 21:791–805.55. Yenari MA, Minami M, Sun GH, et al. Calbindin d28k overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke. 2001. 32:1028–1035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebellum of the nNOS knock-out(-/-) mice
- Changes of Calbindin -D28k Expression Levels After Transient Ischemic Damage in Rat Cerebellar Purkinje Cells
- Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebral cortex and in the hippocampal region of nNOS knock-out(-/-) mice
- Immunocytochemical Study of Calcium Binding Protein in the Distal Nephron of Rat Kidney
- The distribution of calbindin-D28k, parvalbumin, and calretinin immunoreactivity in the inferior colliculus of circling mouse