Yonsei Med J.
2020 Feb;61(2):145-153. 10.3349/ymj.2020.61.2.145.
Culture-Positive Spontaneous Ascitic Infection in Patients with Acute Decompensated Cirrhosis: Multidrug-Resistant Pathogens and Antibiotic Strategies
- Affiliations
-
- 1Department of Infectious Diseases, Institute of Infection and Immunology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. xin11@hotmail.com
- 2Chinese Acute-on-Chronic Liver Failure Consortium, CATCH-LIFE, Shanghai, China.
- 3Department of Hepatology, The First Hospital of Jilin University, Changchun, China.
- 4Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China.
- 5Department of Liver Intensive Care Unit, Shanghai Public Health Clinical Centre, Fudan University, Shanghai, China.
- 6Hepatology Unit, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China.
- 7Department of Infectious Disease, Hunan Key Laboratory of Viral Hepatitis, Xiangya Hospital, Central South University, Changsha, China.
- 8Department of Infectious Disease, Taihe Hospital, Hubei University of Medicine, Shiyan, China.
- 9Liver Disease Center, First Affiliated Hospital of Xinjiang Medical University, Urumuqi, China.
- 10Department of Infectious Diseases, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
- 11Department of Infectious Diseases and Hepatology, The Second Hospital of Shandong University, Jinan, China.
- 12School of Medicine and Health Management, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China.
- 13Department of Gastroenterology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
- KMID: 2468489
- DOI: http://doi.org/10.3349/ymj.2020.61.2.145
Abstract
- PURPOSE
This study investigated multidrug-resistant (MDR) pathogens and antibiotic strategies of culture-positive spontaneous ascitic infection (SAI) in patients with acute decompensated cirrhosis.
MATERIALS AND METHODS
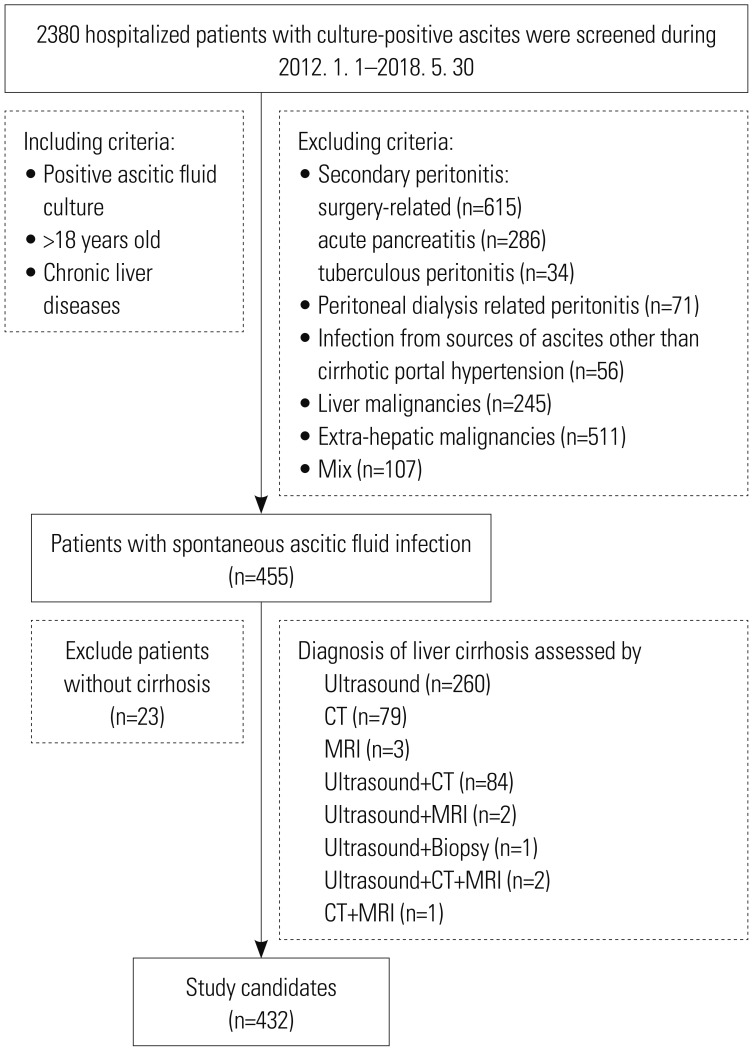
We retrospectively analyzed 432 acute decompensated cirrhotic patients with culture-positive SAI from 11 teaching hospitals in China (January 2012 to May 2018). A Cox proportional hazards model analysis was conducted to identify independent predictors of 28-day mortality.
RESULTS
A total of 455 strains were isolated from 432 ascitic culture samples. Gram-negative bacteria (GNB), gram-positive bacteria (GPB), and fungi caused 52.3, 45.5, and 2.2% of all SAI episodes, respectively. Episodes were classified as nosocomial (41.2%), healthcare-related (34.7%), and community-acquired (24.1%). Escherichia coli (13.4%) and Klebsiella pneumoniae (2.4%) were extended-spectrum β-lactamase producing isolates. The prevalence of methicillin-resistant Staphylococcus aureus was 1.1%. Ceftazidime, cefepime, aztreonam, and amikacin were recommended as first-line antibiotics agents for non-MDR GNB infections; piperacillin/tazobactam and carbapenems for MDR GNB in community-acquired and healthcare-related or nosocomial infections, respectively; and vancomycin or linezolid for GPB infections, regardless of drug-resistance status. Multivariate analysis revealed days of hospital stay before SAI, upper gastrointestinal bleeding, white blood cell count, alanine aminotransferase, serum creatinine concentration, total bilirubin, and international normalized ratio as key independent predictors of 28-day mortality.
CONCLUSION
MDR pathogens and antibiotic strategies were identified in patients with acute decompensated cirrhosis with culture-positive SAI, which may help optimize therapy and improve clinical outcomes.
Keyword
MeSH Terms
-
Alanine Transaminase
Amikacin
Anti-Bacterial Agents
Aztreonam
Bilirubin
Carbapenems
Ceftazidime
China
Creatinine
Cross Infection
Escherichia coli
Fibrosis*
Fungi
Gram-Negative Bacteria
Gram-Positive Bacteria
Hemorrhage
Hospitals, Teaching
Humans
International Normalized Ratio
Klebsiella pneumoniae
Length of Stay
Leukocyte Count
Linezolid
Methicillin-Resistant Staphylococcus aureus
Mortality
Multivariate Analysis
Prevalence
Proportional Hazards Models
Retrospective Studies
Risk Factors
Vancomycin
Alanine Transaminase
Amikacin
Anti-Bacterial Agents
Aztreonam
Bilirubin
Carbapenems
Ceftazidime
Creatinine
Linezolid
Vancomycin
Figure
Reference
-
1. Schwabl P, Bucsics T, Soucek K, Mandorfer M, Bota S, Blacky A, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015; 35:2121–2128. PMID: 25644943.
Article2. Garcia-Tsao G. Bacterial infections in cirrhosis. Can J Gastroenterol. 2004; 18:405–406. PMID: 15190398.
Article3. Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med. 2012; 33:80–95. PMID: 22447263.
Article4. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144:1426–1437. PMID: 23474284.
Article5. Kaymakoglu S, Eraksoy H, Okten A, Demir K, Calangu S, Cakaloglu Y, et al. Spontaneous ascitic infection in different cirrhotic groups: prevalence, risk factors and the efficacy of cefotaxime therapy. Eur J Gastroenterol Hepatol. 1997; 9:71–76. PMID: 9031903.
Article6. Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000; 32:142–153. PMID: 10673079.7. Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010; 139:1246–1256. PMID: 20558165.
Article8. Oey RC, van Buuren HR, de Jong DM, Erler NS, de Man RA. Bacterascites: a study of clinical features, microbiological findings, and clinical significance. Liver Int. 2018; 38:2199–2209. PMID: 29992711.
Article9. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008; 36:296–327. PMID: 18158437.
Article10. Ricart E, Soriano G, Novella MT, Ortiz J, Sàbat M, Kolle L, et al. Amoxicillin-clavulanic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patients. J Hepatol. 2000; 32:596–602. PMID: 10782908.
Article11. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010; 53:397–417. PMID: 20633946.12. Alexopoulou A, Papadopoulos N, Eliopoulos DG, Alexaki A, Tsiriga A, Toutouza M, et al. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013; 33:975–981. PMID: 23522099.
Article13. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018; 69:406–460. PMID: 29653741.14. Goossens H, Ferech M, Vander Stichele R, Elseviers M. ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005; 365:579–587. PMID: 15708101.
Article15. Hung TH, Tsai CC, Hsieh YH, Tsai CC, Tseng CW, Tseng KC. The effect of the first spontaneous bacterial peritonitis event on the mortality of cirrhotic patients with ascites: a nationwide populationbased study in Taiwan. Gut Liver. 2016; 10:803–807. PMID: 27563023.
Article16. Karvellas CJ, Abraldes JG, Arabi YM, Kumar A. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015; 41:747–757. PMID: 25703246.17. Kim JJ, Tsukamoto MM, Mathur AK, Ghomri YM, Hou LA, Sheibani S, et al. Delayed paracentesis is associated with increased inhospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014; 109:1436–1442. PMID: 25091061.
Article18. Lutz P, Nischalke HD, Krämer B, Goeser F, Kaczmarek DJ, Schlabe S, et al. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017; 47:44–52. PMID: 27861767.
Article19. Salerno F, Borzio M, Pedicino C, Simonetti R, Rossini A, Boccia S, et al. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017; 37:71–79. PMID: 27364035.
Article20. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–281. PMID: 21793988.
Article21. CLSI. CLSI supplement M100. Performance standards for antimicrobial susceptibility testing. 28th ed. Wayne (PA): Clinical and Laboratory Standards Institute;2018.22. Ning NZ, Li T, Zhang JL, Qu F, Huang J, Liu X, et al. Clinical and bacteriological features and prognosis of ascitic fluid infection in Chinese patients with cirrhosis. BMC Infect Dis. 2018; 18:253. PMID: 29866104.
Article23. Friedrich K, Nüssle S, Rehlen T, Stremmel W, Mischnik A, Eisenbach C. Microbiology and resistance in first episodes of spontaneous bacterial peritonitis: implications for management and prognosis. J Gastroenterol Hepatol. 2016; 31:1191–1195. PMID: 26676553.
Article24. Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, et al. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009; 48:1230–1236. PMID: 19302016.
Article25. Salerno F, Cazzaniga M. Therapeutic strategies and emergence of multiresistant bacterial strains. Intern Emerg Med. 2010; 5 Suppl 1:S45–S51. PMID: 20865474.
Article26. Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019; 70:398–411. PMID: 30391380.
Article27. Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019; 156:1368–1380. PMID: 30552895.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Ascitic Fluid Culture Methods for Diagnosing Spontaneous Bacterial Peritonitis
- A Prospective, Comparative Study of Two Methods of Ascitic Fluid Culture to Diagnose Spontaneous Bacterial Peritonitis.
- Strategies to combat Gram-negative bacterial resistance to conventional antibacterial drugs: a review
- Risk factors for ascitic fluid infection in cirrhotic patients with variceal bleeding
- Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications