Allergy Asthma Immunol Res.
2020 Mar;12(2):274-291. 10.4168/aair.2020.12.2.274.
Evidence for the Presence of Long-Lived Plasma Cells in Nasal Polyps
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Guangzhou Women and Children's Medical Center, Guangzhou, China.
- 2Department of Otolaryngology-Head and Neck Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. zhengliuent@hotmail.com
- 3Department of Otolaryngology-Head and Neck Surgery, Shenzhen Hospital, Southern Medical University, Shenzhen, China.
- KMID: 2468462
- DOI: http://doi.org/10.4168/aair.2020.12.2.274
Abstract
- PURPOSE
Plasma cells and immunoglobulins (Igs) play a pivotal role in the induction and maintenance of chronic inflammation in nasal polyps. During secondary immune responses, plasma cell survival and Ig production are regulated by the local environment. The purpose of the present study was to investigate the presence of long-lived plasma cells (LLPCs) and specific survival niches for LLPCs in human nasal polyps.
METHODS
Nasal mucosal samples were cultured with an air-liquid interface system and the Ig levels in culture supernatants were analyzed by enzyme-linked immunosorbent assay. The characteristics of LLPCs in nasal polyps were determined by immunohistochemistry and immunofluorescence. The expression of neurotrophins as well as their receptors was detected by quantitative real-time polymerase chain reaction, immunohistochemistry, immunofluorescence, and Western blotting.
RESULTS
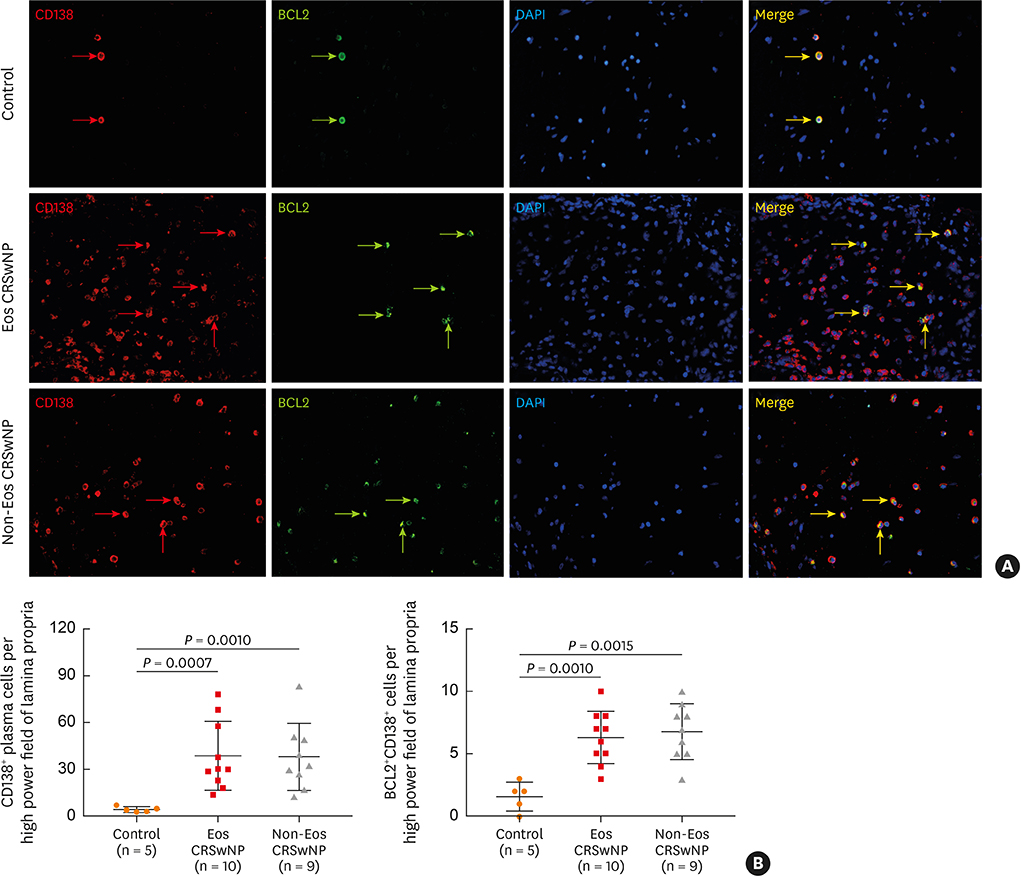
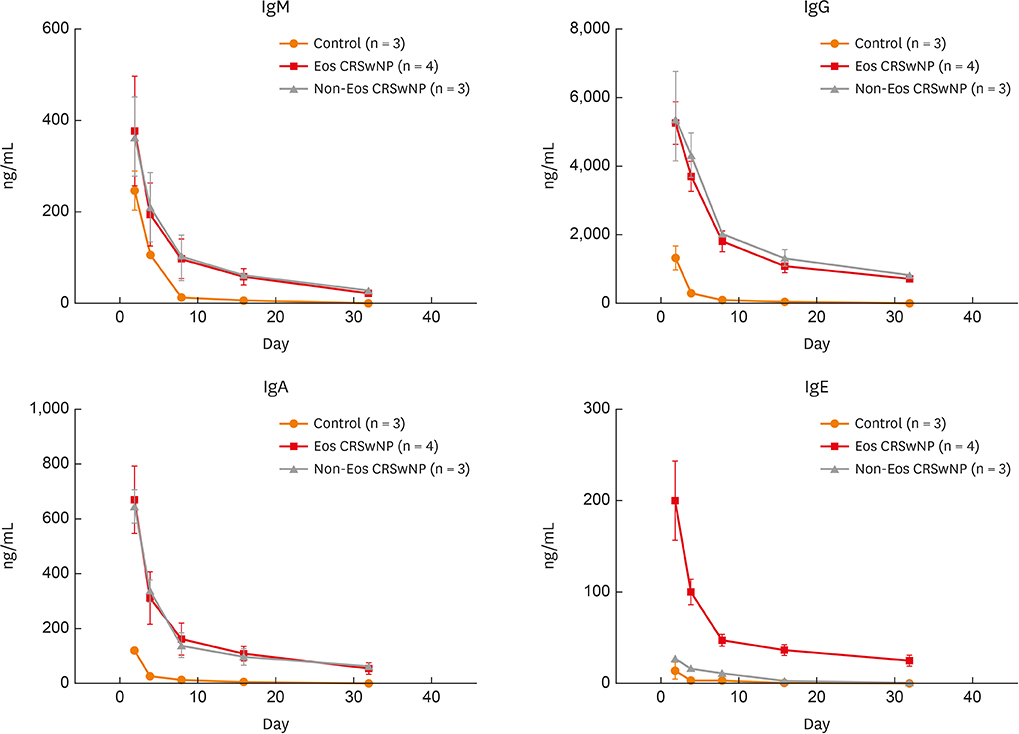
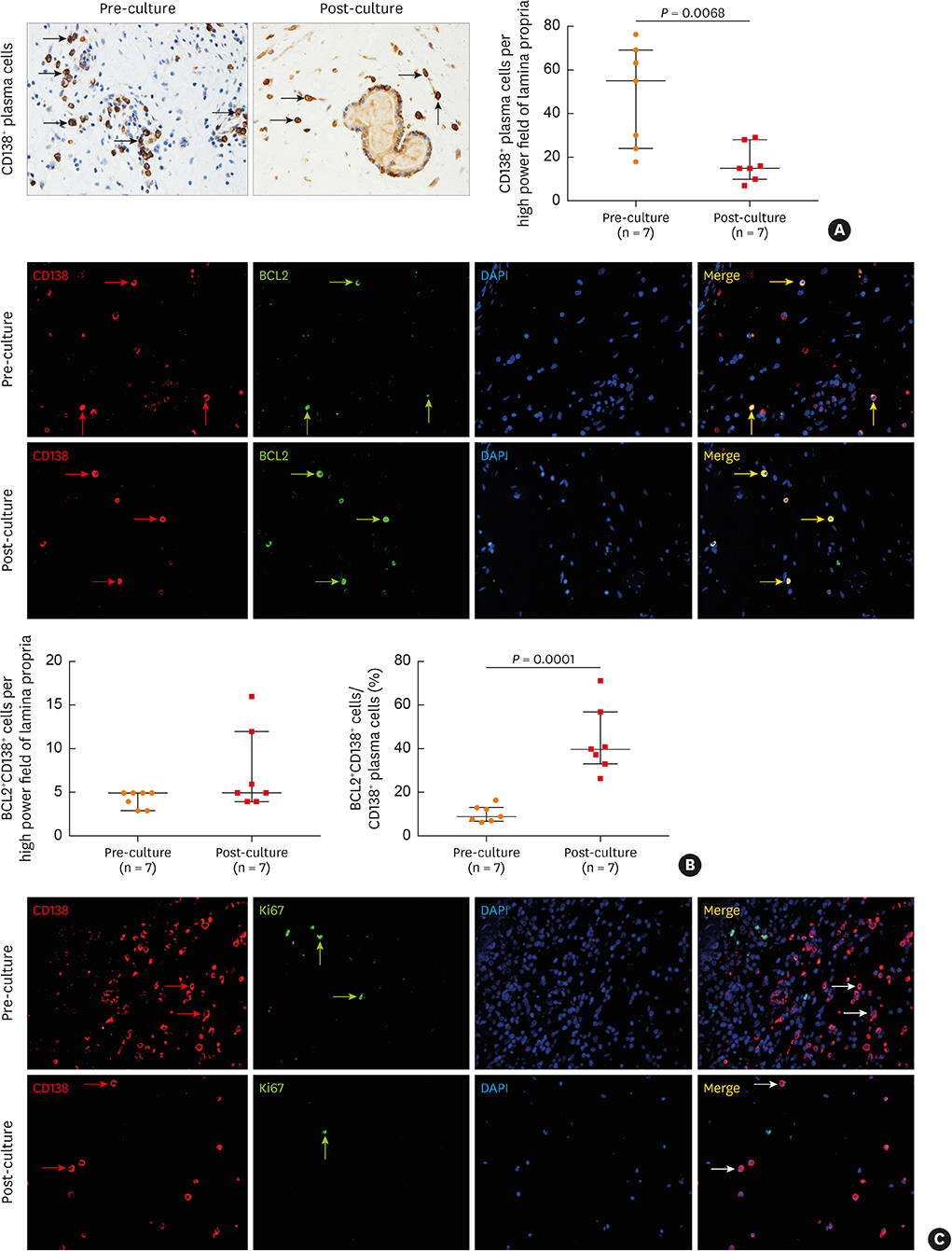
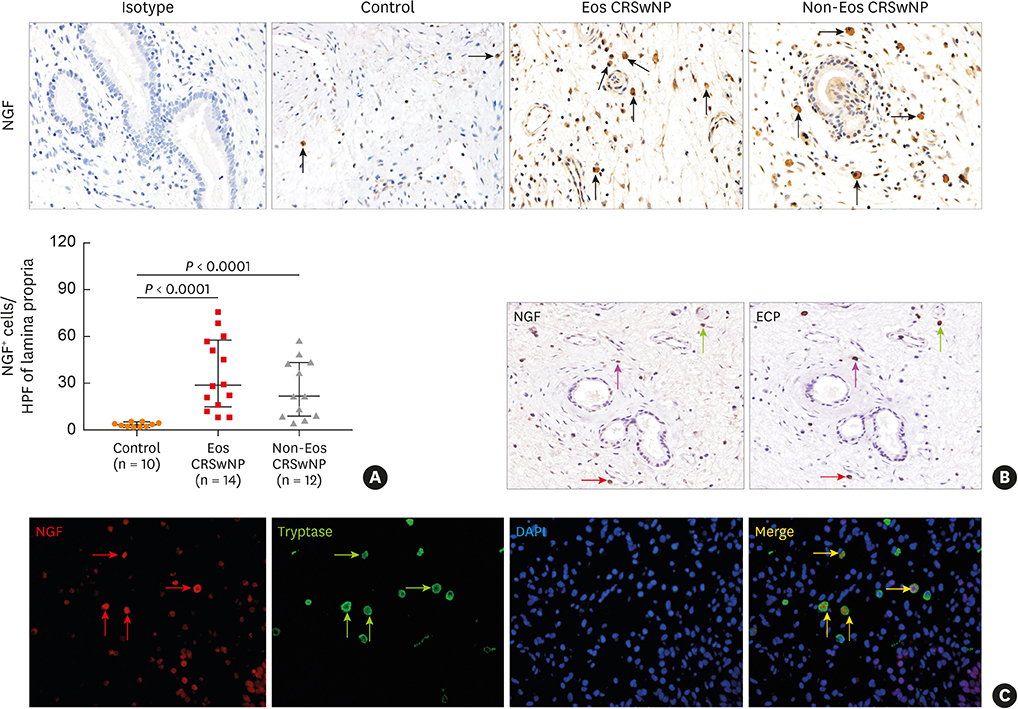
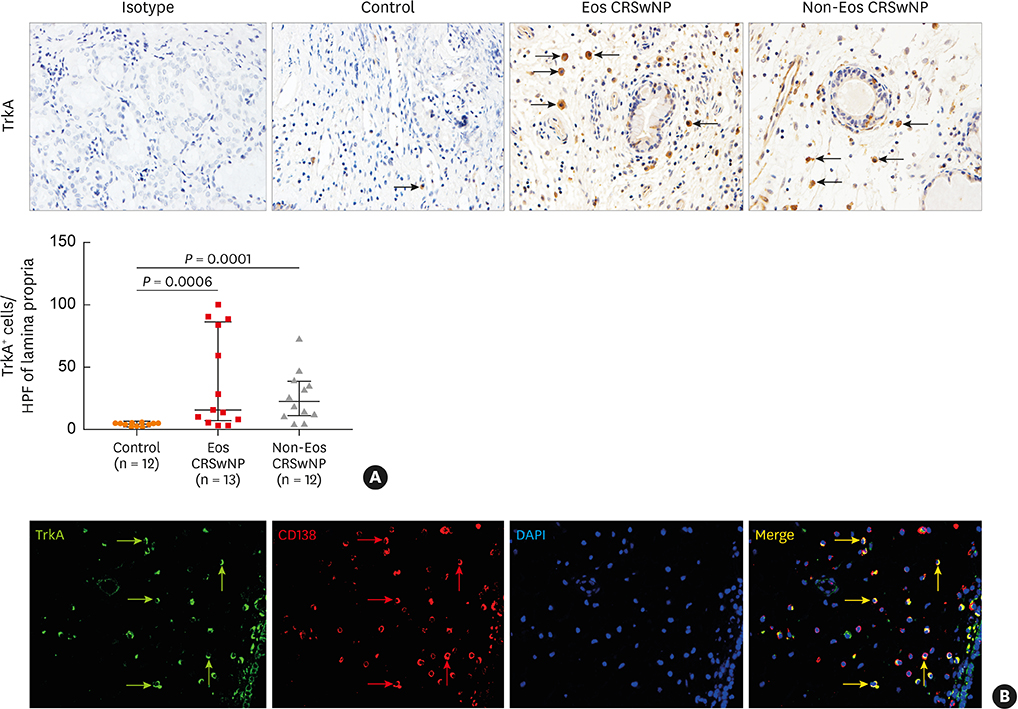
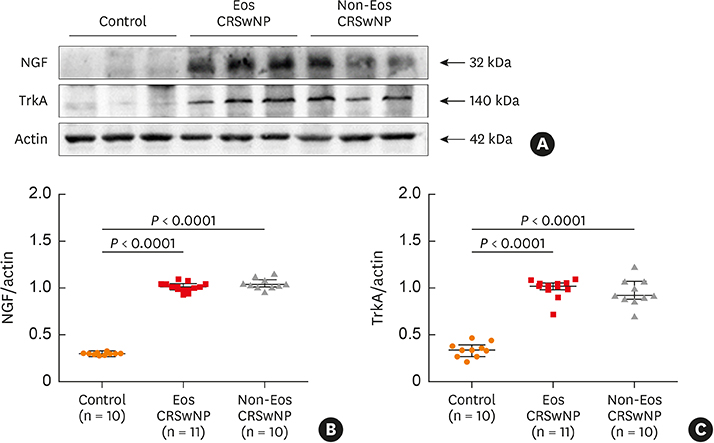
The numbers of CD138⺠total plasma cells and BCL2⺠plasma cells were increased in both eosinophilic and non-eosinophilic nasal polyps compared with those in normal tissues. The production of IgG, IgA, and IgE was detected in culture supernatants even after a 32-day culture of nasal polyps. Although the total numbers of plasma cells were decreased in nasal polyps after culture, the numbers of BCL2⺠plasma cells remained stable. The expression of nerve growth factor (NGF) as well as tropomyosin receptor kinase (Trk) A, a high-affinity receptor for NGF, was upregulated in both eosinophilic and non-eosinophilic nasal polyps. In addition, BCL2⺠plasma cell numbers were positively correlated with NGF and TrkA mRNA expression in nasal mucosal tissues. Polyp plasma cells had the expression of TrkA.
CONCLUSIONS
Human nasal polyps harbor a population of LLPCs and NGF may be involved in their prolonged survival. LLPCs may be a novel therapeutic target for suppressing the local Ig production in nasal polyps.
MeSH Terms
-
Blotting, Western
Enzyme-Linked Immunosorbent Assay
Eosinophils
Fluorescent Antibody Technique
Humans
Immunoglobulin A
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Immunohistochemistry
Inflammation
Mucous Membrane
Nasal Polyps*
Nerve Growth Factor
Nerve Growth Factors
Phosphotransferases
Plasma Cells*
Plasma*
Polyps
Real-Time Polymerase Chain Reaction
RNA, Messenger
Tropomyosin
Immunoglobulin A
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Nerve Growth Factor
Nerve Growth Factors
Phosphotransferases
RNA, Messenger
Tropomyosin
Figure
Cited by 1 articles
-
Impact of the Long-Lived Plasma Cells in Patients With Chronic Rhinosinusitis With Nasal Polyps
Roza Khalmuratova, Hyun-Woo Shin
Allergy Asthma Immunol Res. 2020;12(2):173-175. doi: 10.4168/aair.2020.12.2.173.
Reference
-
1. Luger EO, Fokuhl V, Wegmann M, Abram M, Tillack K, Achatz G, et al. Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol. 2009; 124:819–826.e4.
Article2. van Laar JM, Melchers M, Teng YK, van der Zouwen B, Mohammadi R, Fischer R, et al. Sustained secretion of immunoglobulin by long-lived human tonsil plasma cells. Am J Pathol. 2007; 171:917–927.
Article3. Mesin L, Di Niro R, Thompson KM, Lundin KE, Sollid LM. Long-lived plasma cells from human small intestine biopsies secrete immunoglobulins for many weeks in vitro. J Immunol. 2011; 187:2867–2874.4. Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-lived plasma cells are contained within the CD19−CD38hiCD138+ subset in human bone marrow. Immunity. 2015; 43:132–145.5. Cocco M, Stephenson S, Care MA, Newton D, Barnes NA, Davison A, et al. In vitro generation of long-lived human plasma cells. J Immunol. 2012; 189:5773–5785.6. Nguyen DC, Garimalla S, Xiao H, Kyu S, Albizua I, Galipeau J, et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun. 2018; 9:3698.
Article7. Tellier J, Kallies A. Finding a home for plasma cells--a niche to survive. Eur J Immunol. 2014; 44:2243–2246.8. Minges Wols HA, Ippolito JA, Yu Z, Palmer JL, White FA, Le PT, et al. The effects of microenvironment and internal programming on plasma cell survival. Int Immunol. 2007; 19:837–846.
Article9. Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998; 8:363–372.
Article10. Corcoran LM, Nutt SL. Long-lived plasma cells have a sweet tooth. Immunity. 2016; 45:3–5.
Article11. Peperzak V, Vikström I, Walker J, Glaser SP, LePage M, Coquery CM, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013; 14:290–297.
Article12. Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006; 6:741–750.
Article13. Abram M, Wegmann M, Fokuhl V, Sonar S, Luger EO, Kerzel S, et al. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J Immunol. 2009; 182:4705–4712.
Article14. Taddeo A, Khodadadi L, Voigt C, Mumtaz IM, Cheng Q, Moser K, et al. Long-lived plasma cells are early and constantly generated in New Zealand Black/New Zealand White F1 mice and their therapeutic depletion requires a combined targeting of autoreactive plasma cells and their precursors. Arthritis Res Ther. 2015; 17:39.
Article15. Schuhmann B, Dietrich A, Sel S, Hahn C, Klingenspor M, Lommatzsch M, et al. A role for brain-derived neurotrophic factor in B cell development. J Neuroimmunol. 2005; 163:15–23.
Article16. Kimata H, Yoshida A, Ishioka C, Kusunoki T, Hosoi S, Mikawa H. Nerve growth factor specifically induces human IgG4 production. Eur J Immunol. 1991; 21:137–141.
Article17. Bayas A, Kruse N, Moriabadi NF, Weber F, Hummel V, Wohleben G, et al. Modulation of cytokine mRNA expression by brain-derived neurotrophic factor and nerve growth factor in human immune cells. Neurosci Lett. 2003; 335:155–158.
Article18. Nassenstein C, Braun A, Erpenbeck VJ, Lommatzsch M, Schmidt S, Krug N, et al. The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J Exp Med. 2003; 198:455–467.
Article19. Hiepe F, Dörner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011; 7:170–178.
Article20. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012; 23:3 p preceding table of contents1–298.21. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; 124:478–484. 484.e1–472.
Article22. Zhang YN, Song J, Wang H, Wang H, Zeng M, Zhai GT, et al. Nasal IL-4+CXCR5+CD4+ T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2016; 137:462–473.23. Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011; 128:1198–1206.e1.
Article24. Song J, Wang H, Zhang YN, Cao PP, Liao B, Wang ZZ, et al. Ectopic lymphoid tissues support local immunoglobulin production in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018; 141:927–937.
Article25. Zhai GT, Wang H, Li JX, Cao PP, Jiang WX, Song J, et al. IgD-activated mast cells induce IgE synthesis in B cells in nasal polyps. J Allergy Clin Immunol. 2018; 142:1489–1499.e23.
Article26. Shamji MH, Thomsen I, Layhadi JA, Kappen J, Holtappels G, Sahiner U, et al. Broad IgG repertoire in patients with chronic rhinosinusitis with nasal polyps regulates proinflammatory IgE responses. J Allergy Clin Immunol. 2019; 143:2086–2094.e2.
Article27. Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013; 131:1075–1083. 1083.e1–1077.
Article28. Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007; 37:1840–1847.
Article29. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.
Article30. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, AllerGen, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.31. Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid-induced leucine zipper. Clin Exp Allergy. 2009; 39:647–654.
Article32. Jia XL, Li SY, Dang SS, Cheng YA, Zhang X, Wang WJ, et al. Increased expression of chondroitin sulphate proteoglycans in rat hepatocellular carcinoma tissues. World J Gastroenterol. 2012; 18:3962–3976.
Article33. Pereira T, Naik S, Tamgadge A. Quantitative evaluation of macrophage expression using CD68 in oral submucous fibrosis: an immunohistochemical study. Ann Med Health Sci Res. 2015; 5:435–441.
Article34. Wang BF, Cao PP, Wang ZC, Li ZY, Wang ZZ, Ma J, et al. Interferon-γ-induced insufficient autophagy contributes to p62-dependent apoptosis of epithelial cells in chronic rhinosinusitis with nasal polyps. Allergy. 2017; 72:1384–1397.
Article35. Liu JX, Liao B, Yu QH, Wang H, Liu YB, Guo CL, et al. The IL-37-Mex3B-Toll-like receptor 3 axis in epithelial cells in patients with eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2019; S0091-6749(19)30945-5.
Article36. Miguel-García A, Orero T, Matutes E, Carbonell F, Miguel-Sosa A, Linares M, et al. Bcl-2 expression in plasma cells from neoplastic gammopathies and reactive plasmacytosis: a comparative study. Haematologica. 1998; 83:298–304.37. Puthier D, Pellat-Deceunynck C, Barillé S, Robillard N, Rapp MJ, Juge-Morineau N, et al. Differential expression of Bcl-2 in human plasma cell disorders according to proliferation status and malignancy. Leukemia. 1999; 13:289–294.
Article38. Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006; 117:787–794.
Article39. Nassenstein C, Braun A, Nockher WA, Renz H. Neurotrophin effects on eosinophils in allergic inflammation. Curr Allergy Asthma Rep. 2005; 5:204–211.
Article40. Wu X, Myers AC, Goldstone AC, Togias A, Sanico AM. Localization of nerve growth factor and its receptors in the human nasal mucosa. J Allergy Clin Immunol. 2006; 118:428–433.
Article