J Dent Anesth Pain Med.
2019 Dec;19(6):343-351. 10.17245/jdapm.2019.19.6.343.
Effects of remifentanil preconditioning on factors related to uterine contraction in WISH cells
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Korea. uzleen@me.com
- 2Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea.
- KMID: 2466649
- DOI: http://doi.org/10.17245/jdapm.2019.19.6.343
Abstract
- BACKGROUND
Preterm labor and miscarriage may occur in stressful situations, such as a surgical operation or infection during pregnancy. Pharyngeal and buccal abscess and facial bone fractures are inevitable dental surgeries in pregnant patients. Remifentanil is an opioid analgesic that is commonly used for general anesthesia and sedation. Nonetheless, no study has investigated the effects of remifentanil on amniotic epithelial cells. This study evaluated the effects of remifentanil on the factors related to uterine contraction and its mechanism of action on amniotic epithelial cells.
METHODS
Amniotic epithelial cells were preconditioned at various concentrations of remifentanil for 1 h, followed by 24-h lipopolysaccharide (LPS) exposure. MTT assays were performed to assess the cell viability in each group. The effects of remifentanil on factors related to uterine contractions in amniotic epithelial cells were assessed using a nitric oxide (NO) assay, western blot examinations of the expression of nuclear factor-kappa B (NF-κB), cyclooxygenase 2 (COX2), and prostaglandin E2 (PGE₂), and RT-PCR examinations of the expression of the proinflammatory cytokines interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α).
RESULTS
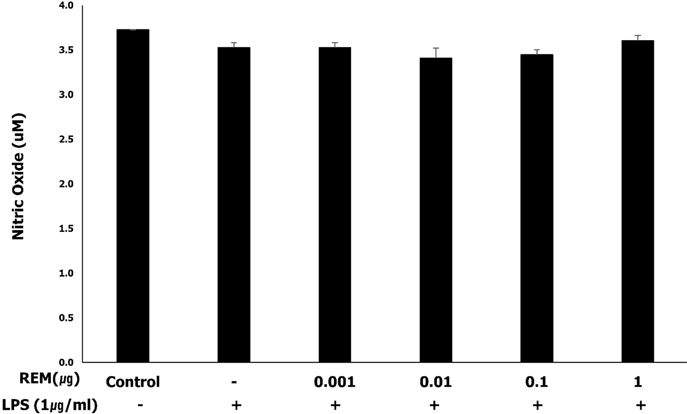
Remifentanil did not affect viability and nitric oxide production of amniotic epithelial cells. Western blot analysis revealed that remifentanil preconditioning resulted in decreased expressions of NF-κB and PGE2 in the cells in LPS-induced inflammation, and a tendency of decreased COX2 expression. The results were statistically significant only at high concentration. RT-PCR revealed reduced expressions of IL-1β and TNF-α.
CONCLUSION
Preconditioning with remifentanil does not affect the viability of amniotic epithelial cells but reduces the expression of factors related to uterine contractions in situations where cell inflammation is induced by LPS, which is an important inducer of preterm labor. These findings provide evidence that remifentanil may inhibit preterm labor in clinical settings.
Keyword
MeSH Terms
-
Abortion, Spontaneous
Abscess
Anesthesia, General
Blotting, Western
Cell Survival
Cyclooxygenase 2
Cytokines
Dinoprostone
Epithelial Cells
Facial Bones
Female
Humans
Inflammation
Interleukins
Lipopolysaccharides
NF-kappa B
Nitric Oxide
Obstetric Labor, Premature
Pregnancy
Tumor Necrosis Factor-alpha
Uterine Contraction*
Cyclooxygenase 2
Cytokines
Dinoprostone
Interleukins
Lipopolysaccharides
NF-kappa B
Nitric Oxide
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000; 342:1500–1507.
Article2. Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004; 104:727–733.
Article3. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009; 16:206–215.
Article4. Kort B, Katz VL, Watson WJ. The effect of nonobstetric operation during pregnancy. Surg Gynecol Obstet. 1993; 177:371–376.
Article5. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008; 371:261–269.
Article6. Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007; 62:1266–1280.
Article7. Maul H, Longo M, Saade GR, Garfield RE. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des. 2003; 9:359–380.
Article8. Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013; 12:86.9. Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol. 2002; 30:285–296.10. Tergaonkar V. NFkappaB pathway: A good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006; 38:1647–1653.11. Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007; 55:453–462.
Article12. Girardin E, Grau GE, Dayer JM, Roux-Lombard P, Lambert PH. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988; 319:397–400.
Article13. Bakker R, Pierce S, Myers D. The role of prostaglandins E1 and E2, dinoprostone, and misoprostol in cervical ripening and the induction of labor: A mechanistic approach. Arch Gynecol Obstet. 2017; 296:167–179.
Article14. Dogru K, Dalgic H, Yildiz K, Sezer Z, Madenoglu H. The direct depressant effects of desflurane and sevoflurane on spontaneous contractions of isolated gravid rat myometrium. Int J Obstet Anesth. 2003; 12:74–78.
Article15. Nacitarhan C, Sadan G, Kayacan N, Ertugrul F, Arici G, Karsli B, et al. The effects of opioids, local anesthetics and adjuvants on isolated pregnant rat uterine muscles. Methods Find Exp Clin Pharmacol. 2007; 29:273–276.
Article16. Shin YK, Kim YD, Collea JV. The effect of propofol on isolated human pregnant uterine muscle. Anesthesiology. 1998; 89:105–109.
Article17. Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002; 21:4831–4840.
Article18. Hyejin J, Mei L, Seongheon L, Cheolwon J, Seokjai K, Hongbeom B, et al. Remifentanil attenuates human neutrophils activation induced by lipopolysaccharide. Immunopharmacol Immunotoxicol. 2013; 35:264–271.
Article19. Zhang Y, Du Z, Zhou Q, Wang Y, Li J. Remifentanil attenuates lipopolysaccharide-induced acute lung injury by downregulating the NF-κB signaling pathway. Inflammation. 2014; 37:1654–1660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effect of remifentanil in lipopolysaccharide–stimulated amniotic epithelial cells
- The Effect of Hypoxic-Preconditioning on the Reperfusion-Induced Arrhythmias in the Cat Hearts
- Remifentanil induces autophagy and prevents hydrogen peroxide-induced apoptosis in Cos-7 cells
- Mechanisms and Prospects of Ischemic Tolerance Induced by Cerebral Preconditioning
- Clinical evaluation of animal research in obstetric anesthesia