J Dent Anesth Pain Med.
2019 Oct;19(5):253-260. 10.17245/jdapm.2019.19.5.253.
Anti-inflammatory effect of remifentanil in lipopolysaccharide–stimulated amniotic epithelial cells
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Korea. anji1030@naver.com
- 2Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea.
- 3Department of Integrated Biological Science, Pusan National University, Busan, Korea.
- KMID: 2461241
- DOI: http://doi.org/10.17245/jdapm.2019.19.5.253
Abstract
- BACKGROUND
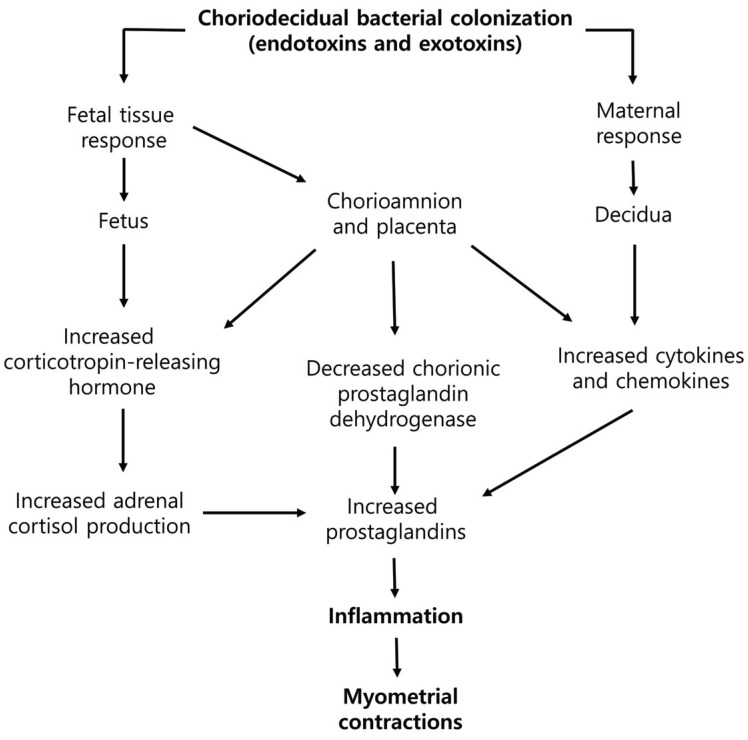
Sometimes general anesthesia is required for dental surgery in pregnant women. Facial bone fractures or neck abscess should be treated immediately. Dental surgery, however, creates a stressful situation that can cause inflammation. Inflammatory responses are a well-known major cause of preterm labor and preterm birth. Here we demonstrate the effects of remifentanil on the factors related to preterm labor and its mechanism of action on amniotic-derived epithelial cells (WISH cells).
METHODS
WISH cells were exposed to lipopolysaccharide (LPS) for 24 h and co-treated with various concentrations of remifentanil. MTT assays were performed to measure cell viability. To explain the effects of remifentanil on the factors related to inflammation in WISH cells, activation of nuclear factor kappa B (NF-κB) and p38 and the expression of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, cyclooxygenase (COX)2, and prostaglandin E (PGE)2 were quantified using western blotting and RT-PCR, respectively.
RESULTS
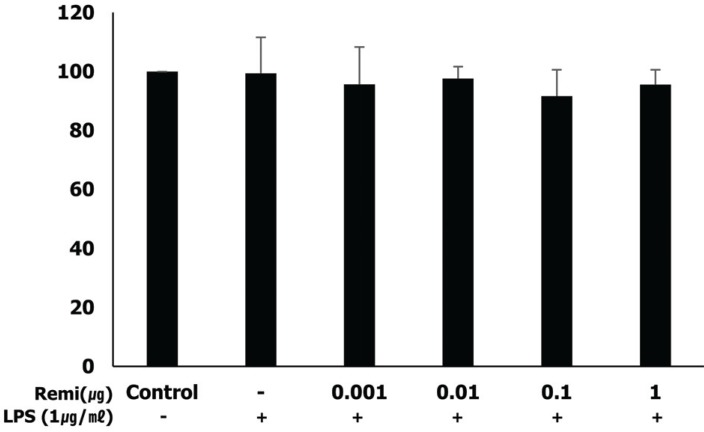
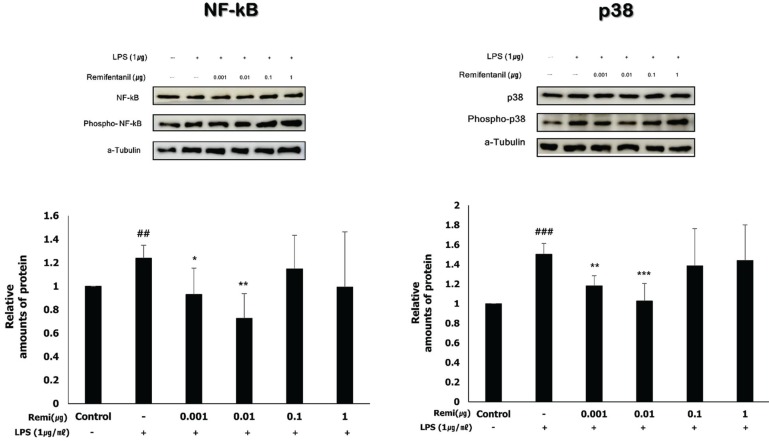
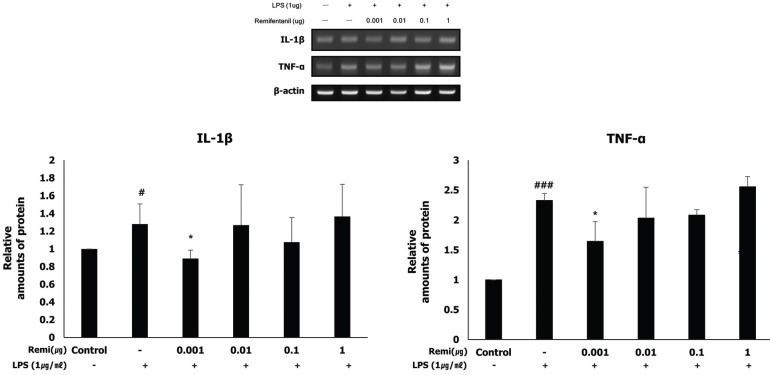
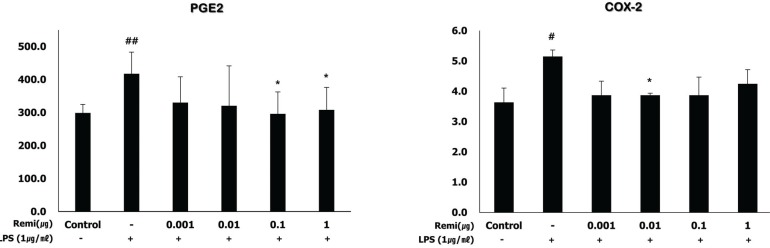
Remifentanil did not affect WISH cell viability. In western blot analysis, co-treatment with remifentanil resulted in decreased phosphorylation of NF-κB, and expression of COX2 and PGE2 in LPS-induced inflammation, but the results were statistically significant only at low concentrations. Reduction of IL-1β and TNF-α expression was also observed with RT-PCR.
CONCLUSION
Co-treatment with remifentanil does not affect the viability of WISH cells, but reduces the expression of the factors related to inflammation, which can induce uterine contraction and preterm labor. These findings provide evidence that remifentanil may inhibit uterine contraction and preterm labor in clinical settings.
Keyword
MeSH Terms
-
Abscess
Amnion
Anesthesia, General
Blotting, Western
Cell Survival
Dinoprostone
Epithelial Cells*
Facial Bones
Female
Humans
Inflammation
Interleukins
Neck
NF-kappa B
Obstetric Labor, Premature
Phosphorylation
Pregnancy
Pregnant Women
Premature Birth
Prostaglandin-Endoperoxide Synthases
Tumor Necrosis Factor-alpha
Uterine Contraction
Dinoprostone
Interleukins
NF-kappa B
Prostaglandin-Endoperoxide Synthases
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008; 371:75–84. PMID: 18177778.
Article2. Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006; 113:17–42.
Article3. Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977; 19:8–12. PMID: 874942.4. Reitman E, Flood P. Anaesthetic considerations for non-obstetric surgery during pregnancy. Br J Anaesth. 2011; 107:i72–i78. PMID: 22156272.
Article5. Hofbauer R, Frass M, Gmeiner B, Sandor N, Schumann R, Wagner O, et al. Effects of remifentanil on neutrophil adhesion, transmigration, and intercellular adhesion molecule expression. Acta Anaesthesiol Scand. 2000; 44:1232–1237. PMID: 11065203.
Article6. Sacerdote P, Gaspani L, Rossoni G, Panerai AE, Bianchi M. Effect of the opioid remifentanil on cellular immune response in the rat. Int Immunopharmacol. 2001; 1:713–719. PMID: 11357883.
Article7. Zhang Y, Du Z, Zhou Q, Wang Y, Li J. Remifentanil attenuates lipopolysaccharide-induced acute lung injury by downregulating the NF-kappaB signaling pathway. Inflammation. 2014; 37:1654–1660. PMID: 24748477.8. Christian F, Smith EL, Carmody RJ. The regulation of NF-kappaB subunits by phosphorylation. Cells. 2016; 5:12.9. Mertens MJ, Olofsen E, Engbers FH, Burm AG, Bovill JG, Vuyk J. Propofol reduces perioperative remifentanil requirements in a synergistic manner: response surface modeling of perioperative remifentanil-propofol interactions. Anesthesiology. 2003; 99:347–359. PMID: 12883407.10. Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007; 62:1266–1280. PMID: 17991265.
Article11. Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996; 60:8–26. PMID: 8699127.
Article12. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988; 31:553–584. PMID: 3066544.
Article13. Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004; 6:1382–1387. PMID: 15596124.
Article14. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994; 12:141–179. PMID: 8011280.15. Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011; 21:71–85. PMID: 21173796.16. Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989; 160:1117–1123. PMID: 2786341.
Article17. Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N Y Acad Sci. 2001; 943:225–234. PMID: 11594542.
Article18. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003; 24:33–46.
Article19. Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989; 37:13–22. PMID: 2785698.20. Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003; 188:252–263. PMID: 12548226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Inflammatory Effects of Fermented Products with Avena sativa on RAW264.7 and HT-29 Cells via Inhibition of Inflammatory Mediators
- Effects of remifentanil preconditioning on factors related to uterine contraction in WISH cells
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- The Effect of Amniotic Membrane on Epithelial Wound Healing in Rabbit Cornea after Phototherapeutic Keratectomy
- Conjunctival Epithelial Cells Auto-Cultivated In Vivo on Human Amniotic Membrane in Rabbits