Lab Anim Res.
2018 Dec;34(4):270-278. 10.5625/lar.2018.34.4.270.
Comparison of the anesthetic effects of 2,2,2-tribromoethanol on ICR mice derived from three different sources
- Affiliations
-
- 1College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. kskim728@knu.ac.kr
- 2Department of Health and Exercise Science, Korea National Sport University, Seoul, Korea.
- 3Biomedical Science Institute, Changwon National University, Changwon, Korea.
- 4Department of Pharmacy, College of Pharmacy, Pusan National University, Busan, Korea.
- 5College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea.
- 6Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- KMID: 2459305
- DOI: http://doi.org/10.5625/lar.2018.34.4.270
Abstract
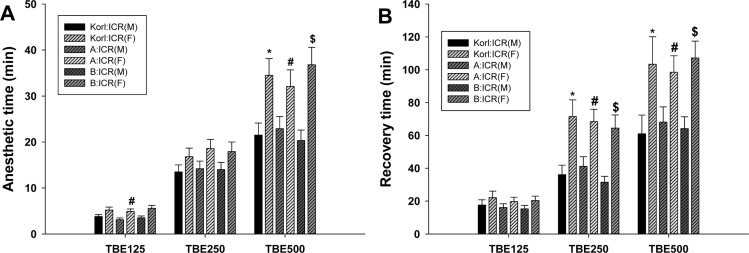
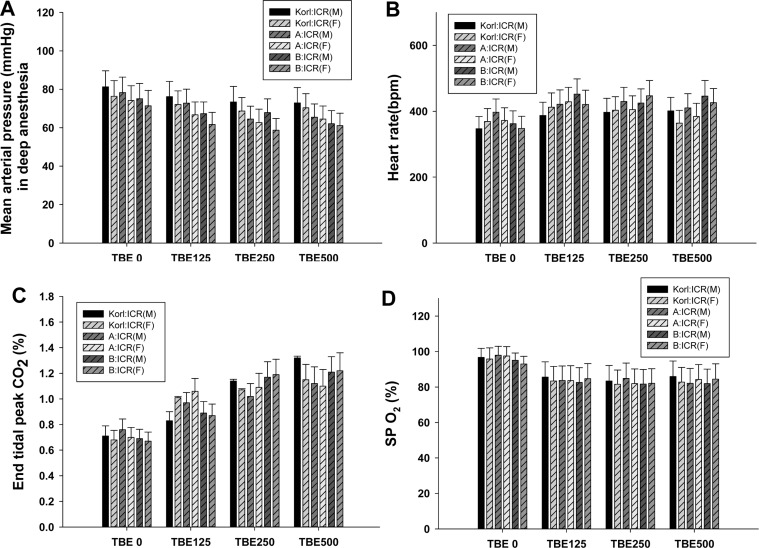
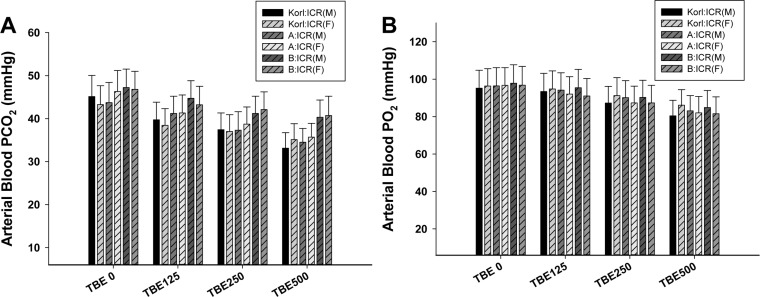
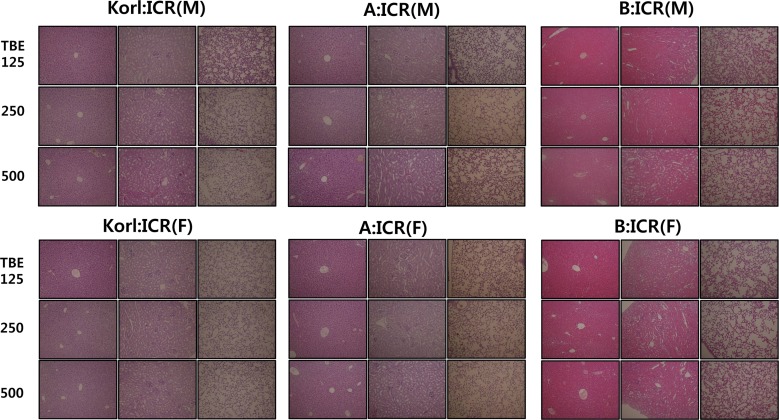
- This study was conducted to compare the anesthetic effects of 2,2,2-tribromoethanol (TBE, Avertin®) in ICR mice obtained from three different sources. TBE (2.5%) was intraperitoneally injected at three doses: high-dose group (500 mg/kg), intermediate-dose group (250 mg/kg), and low-dose group (125 mg/kg). Anesthesia time, recovery time, end-tidal peak CO2 (ETCOâ‚‚), mean arterial blood pressure, heart rate, oxygen saturation (SpOâ‚‚), body temperature, pH, PCOâ‚‚, and POâ‚‚ of the arterial blood were measured. Stable anesthesia was induced by all doses of TBE and the anesthesia time was maintained exhibited dose dependency. No significant differences in anesthetic duration were found among the three different strains. However, the anesthesia time was longer in female than in male mice, and the duration of anesthesia was significantly longer in female than in male mice in the high-dose group. The recovery time was significantly longer for female than male mice in the intermediate- and high-dose groups. In the ICR strains tested, there were no significant differences in the mean arterial blood pressure, SPOâ‚‚, arterial blood PCOâ‚‚, and POâ‚‚, which decreased after TBE anesthesia, or in heart rate and ETCOâ‚‚, which increased after TBE anesthesia. In addition, body temperature, blood biochemical markers, and histopathological changes of the liver, kidney, and lung were not significantly changed by TBE anesthesia. These results suggested that ICR mice from different sources exhibited similar overall responses to a single exposure to TBE anesthesia. In conclusion, TBE is a useful drug that can induce similar anesthetic effects in three different strains of ICR mice.
MeSH Terms
Figure
Reference
-
1. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123. PMID: 8501892.
Article2. Lehoczky JA, Cai WW, Douglas JA, Moran JL, Beier DR, Innis JW. Description and genetic mapping of Polypodia: an X-linked dominant mouse mutant with ectopic caudal limbs and other malformations. Mamm Genome. 2006; 17(9):903–913. PMID: 16964440.
Article3. Al-Awar A, Kupai K, Veszelka M, Szûcs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga C. Experimental Diabetes Mellitus in Different Animal Models. J Diabetes Res. 2016; 2016:9051426. PMID: 27595114.
Article4. Richardson CA, Flecknell PA. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim. 2005; 33(2):119–127. PMID: 16180987.
Article5. Naguib M, Gottumukkala V, Goldstein PA. Melatonin and anesthesia: a clinical perspective. J Pineal Res. 2007; 42(1):12–21. PMID: 17198534.
Article6. Meyer RE, Fish RE. A review of tribromoethanol anesthesia for production of genetically engineered mice and rats. Lab Anim (NY). 2005; 34(10):47–52. PMID: 16261153.
Article7. Hill WA, Tubbs JT, Carter CL, Czarra JA, Newkirk KM, Sparer TE, Rohrbach B, Egger CM. Repeated administration of tribromoethanol in C57BL/6NHsd mice. J Am Assoc Lab Anim Sci. 2013; 52(2):176–179. PMID: 23562101.8. Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993; 43(2):189–192. PMID: 8320967.9. Lieggi CC, Artwohl JE, Leszczynski JK, Rodriguez NA, Fickbohm BL, Fortman JD. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp Top Lab Anim Sci. 2005; 44(1):17–22.10. Voipio HM, Nevalainen T, Virtanen R. Evaluation of anaesthetic potency of medetomidine-ketamine combination in mice. In : IXth ICLAS International Symposium on Laboratory Animal Science Proceedings; Bangkok. 1988. p. 298–299.11. Mulder JB. Anesthesia in the mouse using a combination of ketamine and promazine. Lab Anim Sci. 1978; 28:70–71. PMID: 633840.12. Arras M, Autenried P, Rettich A, Spaeni D, Rülicke T. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med. 2001; 51(5):443–456. PMID: 11924805.13. Wixson SK, White WJ, Hughes HC Jr, Lang CM, Marshall WK. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab Anim Sci. 1987; 37(6):736–742. PMID: 3125387.14. Chu DK, Jordan MC, Kim JK, Couto MA, Roos KP. Comparing isoflurane with tribromoethanol anesthesia for echocardiographic phenotyping of transgenic mice. J Am Assoc Lab Anim Sci. 2006; 45(4):8–13.15. Fish RE. Pharmacology of injectable anesthetics. Anesthesia and Analgesia in Laboratory Animals. New York: Academic Pressv;1997. p. 1–28.16. Weiss J, Zimmermann F. Tribromoethanol (Avertin) as an anaesthetic in mice. Lab Anim. 1999; 33(2):192–193. PMID: 10780824.
Article17. Brown ET, Umino Y, Loi T, Solessio E, Barlow R. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci. 2005; 22(5):615–618. PMID: 16332272.
Article18. Kubo Y, Tahara Y, Hirao A, Shibata S. 2,2,2-Tribromoethanol phase-shifts the circadian rhythm of the liver clock in Per2::Luciferase knockin mice: lack of dependence on anesthetic activity. J Pharmacol Exp Ther. 2012; 340(2):698–705. PMID: 22171092.
Article19. Norton WB, Scavizzi F, Smith CN, Dong W, Raspa M, Parker-Thornburg JV. Refinements for embryo implantation surgery in the mouse: comparison of injectable and inhalant anesthesias - tribromoethanol, ketamine and isoflurane - on pregnancy and pup survival. Lab Anim. 2016; 50(50):335–343. PMID: 26566637.
Article20. Oh SS, Hayes JM, Sims-Robinson C, Sullivan KA, Feldman EL. The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci Lett. 2010; 483(2):127–131. PMID: 20691755.
Article21. Hogan B, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory;1986.22. Buetow BS, Chen LI, Maggio-Price L, Swisshelm K. Peritonitis in Nude Mice in a Xenograft Study. Contemp Top Lab Anim Sci. 1999; 38(6):47–49.23. Kohn DF, Wixson SK, White WJ, Benson GJ. Anesthesia and Analgesia in Laboratory Animals. San Diego: Academic Press;1997.24. Gardner DJ, Davis JA, Weina PJ, Theune B. Comparison of tribromoethanol, ketamine/acetylpromazine, Telazol/xylazine, pentobarbital, and methoxyflurane anesthesia in HSD:ICR mice. Lab Anim Sci. 1995; 45(2):199–204. PMID: 7603025.25. Koizumi T, Maeda H, Hioki K. Sleep-time variation for ethanol and the hypnotic drugs tribromoethanol, urethane, pentobarbital, and propofol within outbred ICR mice. Exp Anim. 2002; 51(2):119–124. PMID: 12012718.
Article26. Flecknell PA. Chapter 3, Analgesic management. Laboratory Animal Anaesthesia. 3rd ed. London: Academic Press;2009.27. Hart CY, Burnett JC Jr, Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol. 2001; 281(5):H1938–H1945. PMID: 11668054.
Article28. Rousselon S, Coat M, Nguyen BV, Gouny P, Nowak E, Wargnier JP, Arvieux CC, Gueret G. [Comparison between ETCO2 values measured by the Smart Capnoline™ and the PACO2 in intubated then extubated postoperative cardiac surgery patients]. Ann Fr Anesth Reanim. 2011; 30(1):13–16. PMID: 21190808.29. American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric advanced life support. Pediatrics. 2006; 117(5):e1005–e1028. PMID: 16651281.30. Benallal H, Busso T. Analysis of end-tidal and arterial PCO2 gradients using a breathing model. Eur J Appl Physiol. 2000; 83(4-5):402–408. PMID: 11138582.31. Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Normal values. Arterial and end-tidal carbon dioxide tensions. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2005.32. Enghoff H. Volumen inefficax: Bemerkungen zur frage des schädlichen raumes. Upsala Lakareforen Forh. 1938; 44:191–218.33. Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002; 282(6):H2134–H2140. PMID: 12003821.34. Lieggi CC, Artwohl JE, Leszczynski JK, Rodriguez NA, Fickbohm BL, Fortman JD. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp Top Lab Anim Sci. 2005; 44(1):17–22.35. Zeller W, Meier G, Bürki K, Panoussis B. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim. 1998; 32(4):407–413. PMID: 9807753.
Article36. Flecknell PA. Chapter 2, Anesthesia. Laboratory Animal Anaesthesia. 3rd ed. London: Academic Press;2009.37. Boyd RL, Halderman LW, Harris JO, Mangos JA. Strain differences in pulmonary function of laboratory rats. Lab Anim Sci. 1982; 32(1):42–43. PMID: 7078071.38. Thompson JS, Brown SA, Khurdayan V, Zeynalzadedan A, Sullivan PG, Scheff SW. Early effects of tribromoethanol, ketamine/xylazine, pentobarbitol, and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp Med. 2002; 52(1):63–67. PMID: 11900415.39. Goelz MF. Anesthetic and pathologic effects of tribromoethanol in mice. Toxicology Bibliographic Information (Toxline);1994.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation on Efficacy and Safety of Tribromoethanol and Tribromoethanol plus alpha2-Adrenergic Agonists in Different Mouse Strains
- Kinetics of proinflammatory cytokines after intraperitoneal injection of tribromoethanol and a tribromoethanol/xylazine combination in ICR mice
- Comparison of the response using ICR mice derived from three different sources to multiple low-dose streptozotocin-induced diabetes mellitus
- Comparison of therapeutic responses to an anticancer drug in three stocks of ICR mice derived from three different sources
- Comparative analysis of basal locomotor activity-related metabolic phenotypes between C57BL/6 mice and ICR mice substrains derived from three different sources